正在加载图片...
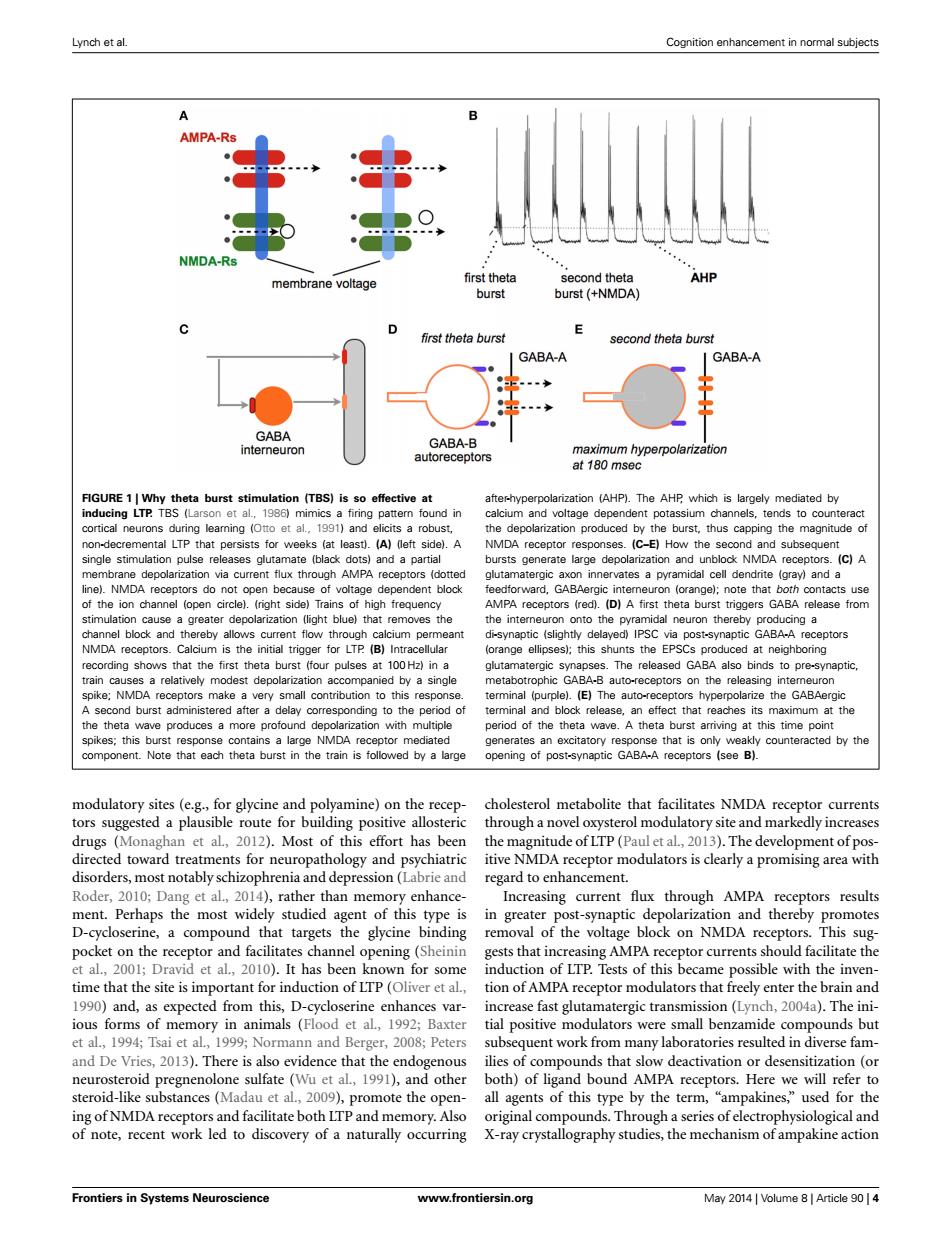
Lynch et al. Cognition enhancement in normal subjects A AMPA-Rs NMDA-Rs AHP membrane voltage first theta second theta burst burst (+NMDA) first theta burst second theta burst GABA-A GABA-A GABA GABA-B interneuron autoreceptors maximum hyperpolarization at 180 msec FIGURE 1|Why theta burst stimulation (TBS)is so effective at after-hyperpolarization (AHP).The AHP which is largely mediated by inducing LTP.TBS (Larson et al.,1986)mimics a firing pattern found in calcium and voltage dependent potassium channels,tends to counteract cortical neurons during leaming (Otto et al..1991)and elicits a robust, the depolarization produced by the burst,thus capping the magnitude of non-decremental LTP that persists for weeks (at least).(A)(left side).A NMDA receptor responses.(C-E)How the second and subsequent single stimulation pulse releases glutamate (black dots)and a partial bursts generate large depolarization and unblock NMDA receptors.(C)A membrane depolarization via current flux through AMPA receptors (dotted glutamatergic axon innervates a pyramidal cell dendrite (gray)and a line).NMDA receptors do not open because of voltage dependent block feedforward,GABAergic interneuron (orange);note that both contacts use of the ion channel (open circle).(right side)Trains of high frequency AMPA receptors (red).(D)A first theta burst triggers GABA release from stimulation cause a greater depolarization (light blue)that removes the the interneuron onto the pyramidal neuron thereby producing a channel block and thereby allows current flow through calcium permeant di-synaptic (slightly delayed)IPSC via post-synaptic GABA-A receptors NMDA receptors.Calcium is the initial trigger for LTP (B)Intracellular (orange ellipses);this shunts the EPSCs produced at neighboring recording shows that the first theta burst (four pulses at 100 Hz)in a glutamatergic synapses.The released GABA also binds to pre-synaptic, train causes a relatively modest depolarization accompanied by a single metabotrophic GABA-B auto-receptors on the releasing intemeuron spike;NMDA receptors make a very small contribution to this response. terminal (purple).(E)The auto-receptors hyperpolarize the GABAergic A second burst administered after a delay corresponding to the period of terminal and block release,an effect that reaches its maximum at the the theta wave produces a more profound depolarization with multiple period of the theta wave.A theta burst arriving at this time point spikes;this burst response contains a large NMDA receptor mediated generates an excitatory response that is only weakly counteracted by the component.Note that each theta burst in the train is followed by a large opening of post-synaptic GABA-A receptors (see B). modulatory sites (e.g.,for glycine and polyamine)on the recep- cholesterol metabolite that facilitates NMDA receptor currents tors suggested a plausible route for building positive allosteric through a novel oxysterol modulatory site and markedly increases drugs (Monaghan et al,2012).Most of this effort has been the magnitude of LTP(Paul et al,2013).The development of pos- directed toward treatments for neuropathology and psychiatric itive NMDA receptor modulators is clearly a promising area with disorders,most notably schizophrenia and depression(Labrie and regard to enhancement. Roder,2010;Dang et al.,2014),rather than memory enhance- Increasing current flux through AMPA receptors results ment.Perhaps the most widely studied agent of this type is in greater post-synaptic depolarization and thereby promotes D-cycloserine,a compound that targets the glycine binding removal of the voltage block on NMDA receptors.This sug- pocket on the receptor and facilitates channel opening(Sheinin gests that increasing AMPA receptor currents should facilitate the et al.,2001;Dravid et al.,2010).It has been known for some induction of LTP.Tests of this became possible with the inven- time that the site is important for induction of LTP(Oliver et al.,tion of AMPA receptor modulators that freely enter the brain and 1990)and,as expected from this,D-cycloserine enhances var- increase fast glutamatergic transmission(Lynch,2004a).The ini- ious forms of memory in animals (Flood et al,1992;Baxter tial positive modulators were small benzamide compounds but et al.,1994;Tsai et al.,1999;Normann and Berger,2008;Peters subsequent work from many laboratories resulted in diverse fam- and De Vries,2013).There is also evidence that the endogenous ilies of compounds that slow deactivation or desensitization (or neurosteroid pregnenolone sulfate (Wu et al,1991),and other both)of ligand bound AMPA receptors.Here we will refer to steroid-like substances(Madau et al.,2009),promote the open- all agents of this type by the term,"ampakines,"used for the ing of NMDA receptors and facilitate both LTP and memory.Also original compounds.Through a series of electrophysiological and of note,recent work led to discovery of a naturally occurring X-ray crystallography studies,the mechanism of ampakine action Frontiers in Systems Neuroscience www.frontiersin.org May 2014 Volume 8 Article 90 4Lynch et al. Cognition enhancement in normal subjects FIGURE 1 | Why theta burst stimulation (TBS) is so effective at inducing LTP. TBS (Larson et al., 1986) mimics a firing pattern found in cortical neurons during learning (Otto et al., 1991) and elicits a robust, non-decremental LTP that persists for weeks (at least). (A) (left side). A single stimulation pulse releases glutamate (black dots) and a partial membrane depolarization via current flux through AMPA receptors (dotted line). NMDA receptors do not open because of voltage dependent block of the ion channel (open circle). (right side) Trains of high frequency stimulation cause a greater depolarization (light blue) that removes the channel block and thereby allows current flow through calcium permeant NMDA receptors. Calcium is the initial trigger for LTP. (B) Intracellular recording shows that the first theta burst (four pulses at 100 Hz) in a train causes a relatively modest depolarization accompanied by a single spike; NMDA receptors make a very small contribution to this response. A second burst administered after a delay corresponding to the period of the theta wave produces a more profound depolarization with multiple spikes; this burst response contains a large NMDA receptor mediated component. Note that each theta burst in the train is followed by a large after-hyperpolarization (AHP). The AHP, which is largely mediated by calcium and voltage dependent potassium channels, tends to counteract the depolarization produced by the burst, thus capping the magnitude of NMDA receptor responses. (C–E) How the second and subsequent bursts generate large depolarization and unblock NMDA receptors. (C) A glutamatergic axon innervates a pyramidal cell dendrite (gray) and a feedforward, GABAergic interneuron (orange); note that both contacts use AMPA receptors (red). (D) A first theta burst triggers GABA release from the interneuron onto the pyramidal neuron thereby producing a di-synaptic (slightly delayed) IPSC via post-synaptic GABA-A receptors (orange ellipses); this shunts the EPSCs produced at neighboring glutamatergic synapses. The released GABA also binds to pre-synaptic, metabotrophic GABA-B auto-receptors on the releasing interneuron terminal (purple). (E) The auto-receptors hyperpolarize the GABAergic terminal and block release, an effect that reaches its maximum at the period of the theta wave. A theta burst arriving at this time point generates an excitatory response that is only weakly counteracted by the opening of post-synaptic GABA-A receptors (see B). modulatory sites (e.g., for glycine and polyamine) on the receptors suggested a plausible route for building positive allosteric drugs (Monaghan et al., 2012). Most of this effort has been directed toward treatments for neuropathology and psychiatric disorders, most notably schizophrenia and depression (Labrie and Roder, 2010; Dang et al., 2014), rather than memory enhancement. Perhaps the most widely studied agent of this type is D-cycloserine, a compound that targets the glycine binding pocket on the receptor and facilitates channel opening (Sheinin et al., 2001; Dravid et al., 2010). It has been known for some time that the site is important for induction of LTP (Oliver et al., 1990) and, as expected from this, D-cycloserine enhances various forms of memory in animals (Flood et al., 1992; Baxter et al., 1994; Tsai et al., 1999; Normann and Berger, 2008; Peters and De Vries, 2013). There is also evidence that the endogenous neurosteroid pregnenolone sulfate (Wu et al., 1991), and other steroid-like substances (Madau et al., 2009), promote the opening of NMDA receptors and facilitate both LTP and memory. Also of note, recent work led to discovery of a naturally occurring cholesterol metabolite that facilitates NMDA receptor currents through a novel oxysterol modulatory site and markedly increases the magnitude of LTP (Paul et al., 2013). The development of positive NMDA receptor modulators is clearly a promising area with regard to enhancement. Increasing current flux through AMPA receptors results in greater post-synaptic depolarization and thereby promotes removal of the voltage block on NMDA receptors. This suggests that increasing AMPA receptor currents should facilitate the induction of LTP. Tests of this became possible with the invention of AMPA receptor modulators that freely enter the brain and increase fast glutamatergic transmission (Lynch, 2004a). The initial positive modulators were small benzamide compounds but subsequent work from many laboratories resulted in diverse families of compounds that slow deactivation or desensitization (or both) of ligand bound AMPA receptors. Here we will refer to all agents of this type by the term, “ampakines,” used for the original compounds. Through a series of electrophysiological and X-ray crystallography studies, the mechanism of ampakine action Frontiers in Systems Neuroscience www.frontiersin.org May 2014 | Volume 8 | Article 90 | 4