正在加载图片...
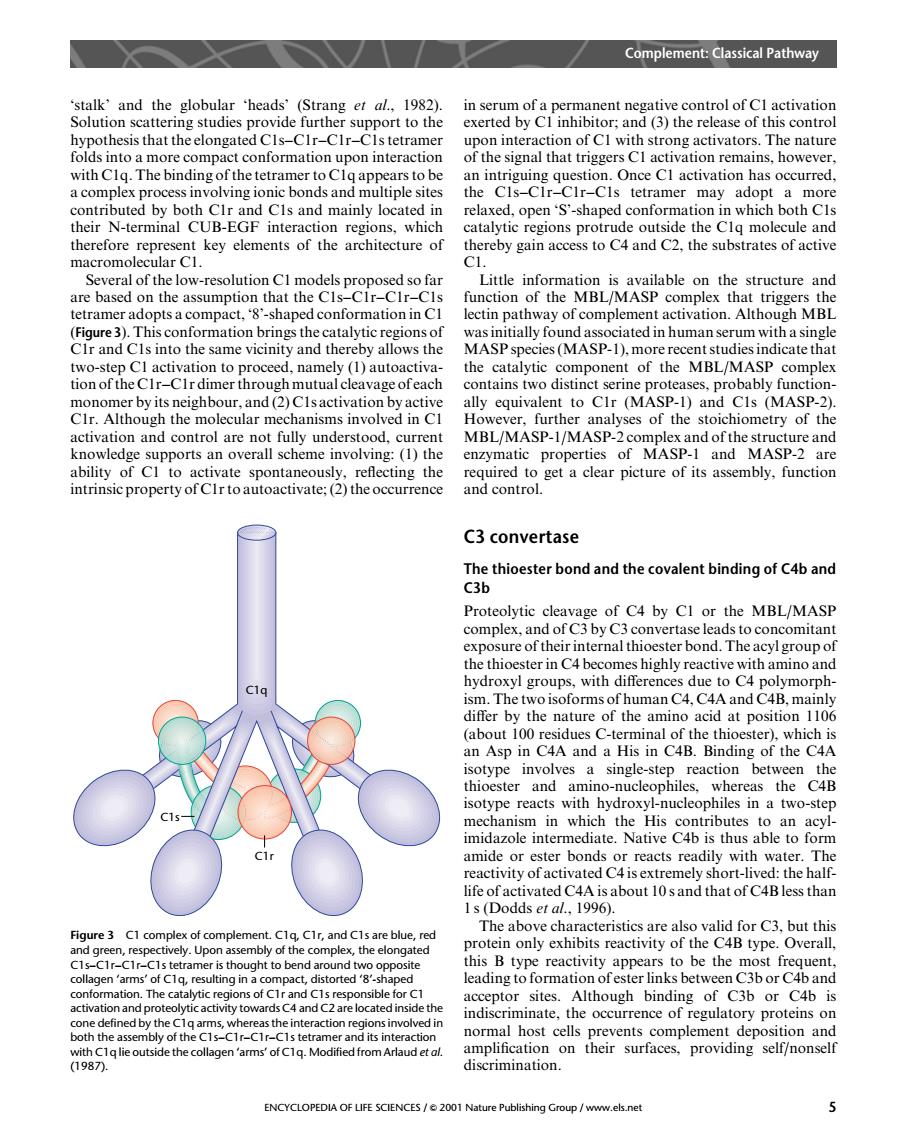
Complement:Classical Pathway activation n of Can h str The natu of the signl that triggers Cl activation remains,howeve a complex pro s an s tetramer may adopt a heir N-terminal CUB-EGF inter reions.which oth C n therefore represent key elements of the architecture of thereby gain access to C4 and C2.the substrates of active macromolec lar C1 everal of the low-resoionCmodpropos structure and of complement activation.Although MBL (Figure3).This conformation brings the catalytic regionsof CIr and CIs into the same vicinity and thereby allows the dicate that MBL/MASP ally equivalent to CIr (MASP-1)and CIs (MASP-2) CIr.Although the molecular mechanisms involved in CI However,further analyses of the stoichiometry of the o Cupports an overal propertie and cear picture of fuctio are C3 convertase Proteolytic complex.hei of C4 by CI or the MBL/MASP nd Th reactive with amino and hydroxyl groups,with differences due to C4 polymorph- ism.The two isoforms of human C4,C4A and C4B,mainl i00 e nature at pos pCAA Ca Ing or th CA sotype involves a single-step reaction between the thioester and a sotype rea amino-nucleophi ich the two-step midazole intermediate.Native 4p is thus able to form amide or ester bonds or reacts readily with water.The eactivity of activated C4isextremely ort- the hal The above characteristics are also valid for c3 but this Figure3 C1 complex of complement.CIr,and C1s are blue, re ghthe com protein only exhibits reactivity of the C4B type.Overall. ytic regions of Cr and C1s resp sible A14 indiscriminate.the occurrence of regulatory proteins on the c normal host cells prevents complement deposition and amplif ENCYCLOPEDIA OF LIFE SCIENCES/2001 Nature Publishing Group /www.els.net ‘stalk’ and the globular ‘heads’ (Strang et al., 1982). Solution scattering studies provide further support to the hypothesis that the elongated C1s–C1r–C1r–C1s tetramer folds into a more compact conformation upon interaction with C1q. The binding of the tetramer to C1q appears to be a complex process involving ionic bonds and multiple sites contributed by both C1r and C1s and mainly located in their N-terminal CUB-EGF interaction regions, which therefore represent key elements of the architecture of macromolecular C1. Several of the low-resolution C1 models proposed so far are based on the assumption that the C1s–C1r–C1r–C1s tetramer adopts a compact, ‘8’-shaped conformation in C1 (Figure 3). This conformation brings the catalytic regions of C1r and C1s into the same vicinity and thereby allows the two-step C1 activation to proceed, namely (1) autoactivation of the C1r–C1r dimer through mutual cleavage of each monomer by its neighbour, and (2) C1s activation by active C1r. Although the molecular mechanisms involved in C1 activation and control are not fully understood, current knowledge supports an overall scheme involving: (1) the ability of C1 to activate spontaneously, reflecting the intrinsic property of C1r to autoactivate; (2) the occurrence in serum of a permanent negative control of C1 activation exerted by C1 inhibitor; and (3) the release of this control upon interaction of C1 with strong activators. The nature of the signal that triggers C1 activation remains, however, an intriguing question. Once C1 activation has occurred, the C1s–C1r–C1r–C1s tetramer may adopt a more relaxed, open ‘S’-shaped conformation in which both C1s catalytic regions protrude outside the C1q molecule and thereby gain access to C4 and C2, the substrates of active C1. Little information is available on the structure and function of the MBL/MASP complex that triggers the lectin pathway of complement activation. Although MBL was initially found associated in human serum with a single MASP species (MASP-1), more recent studies indicate that the catalytic component of the MBL/MASP complex contains two distinct serine proteases, probably functionally equivalent to C1r (MASP-1) and C1s (MASP-2). However, further analyses of the stoichiometry of the MBL/MASP-1/MASP-2 complex and of the structure and enzymatic properties of MASP-1 and MASP-2 are required to get a clear picture of its assembly, function and control. C3 convertase The thioester bond and the covalent binding of C4b and C3b Proteolytic cleavage of C4 by C1 or the MBL/MASP complex, and of C3 by C3 convertase leads to concomitant exposure of their internal thioester bond. The acyl group of the thioester in C4 becomes highly reactive with amino and hydroxyl groups, with differences due to C4 polymorphism. The two isoforms of human C4, C4A and C4B, mainly differ by the nature of the amino acid at position 1106 (about 100 residues C-terminal of the thioester), which is an Asp in C4A and a His in C4B. Binding of the C4A isotype involves a single-step reaction between the thioester and amino-nucleophiles, whereas the C4B isotype reacts with hydroxyl-nucleophiles in a two-step mechanism in which the His contributes to an acylimidazole intermediate. Native C4b is thus able to form amide or ester bonds or reacts readily with water. The reactivity of activated C4 is extremely short-lived: the halflife of activated C4A is about 10 s and that of C4B less than 1 s (Dodds et al., 1996). The above characteristics are also valid for C3, but this protein only exhibits reactivity of the C4B type. Overall, this B type reactivity appears to be the most frequent, leading to formation of ester links between C3b or C4b and acceptor sites. Although binding of C3b or C4b is indiscriminate, the occurrence of regulatory proteins on normal host cells prevents complement deposition and amplification on their surfaces, providing self/nonself discrimination. C1q C1s C1r Figure 3 C1 complex of complement. C1q, C1r, and C1s are blue, red and green, respectively. Upon assembly of the complex, the elongated C1s–C1r–C1r–C1s tetramer is thought to bend around two opposite collagen ‘arms’ of C1q, resulting in a compact, distorted ‘8’-shaped conformation. The catalytic regions of C1r and C1s responsible for C1 activation and proteolytic activity towards C4 and C2 are located inside the cone defined by the C1q arms, whereas the interaction regions involved in both the assembly of the C1s–C1r–C1r–C1s tetramer and its interaction with C1q lie outside the collagen ‘arms’ of C1q. Modified from Arlaud et al. (1987). Complement: Classical Pathway ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 5