正在加载图片...
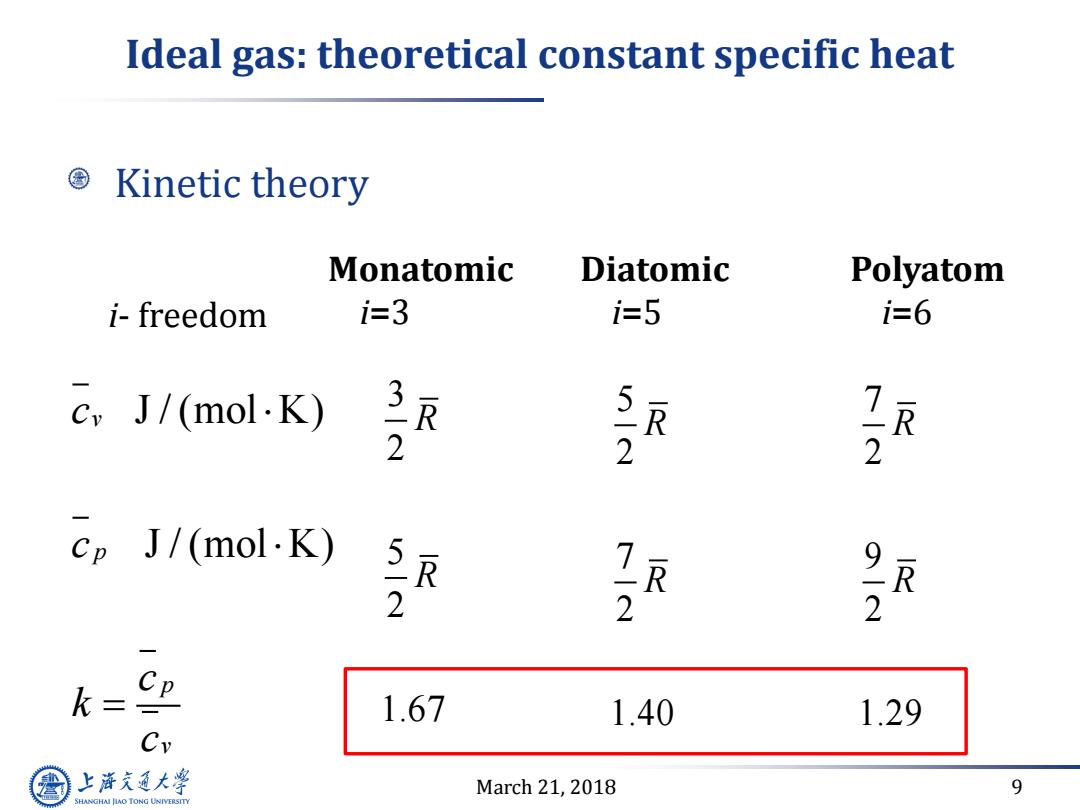
Ideal gas:theoretical constant specific heat Kinetic theory Monatomic Diatomic Polyatom i-freedom i=3 i-5 i=6 cvJ/(mol·K) 7 2 2 2 ep J/(mol.K) R 2 2 2 k= CP 1.67 1.40 1.29 Cy 上游通大学 March 21,2018 9 SHANGHAI JLAO TONG UNIVERSITYMarch 21, 2018 9 Ideal gas: theoretical constant specific heat Kinetic theory Monatomic i=3 Diatomic i=5 Polyatom i=6 J / (mol K) J / (mol K) v p p v c c c k c 3 2 5 2 1.67 R R i- freedom 5 2 7 2 1.40 R R 7 2 9 2 1.29 R R