正在加载图片...
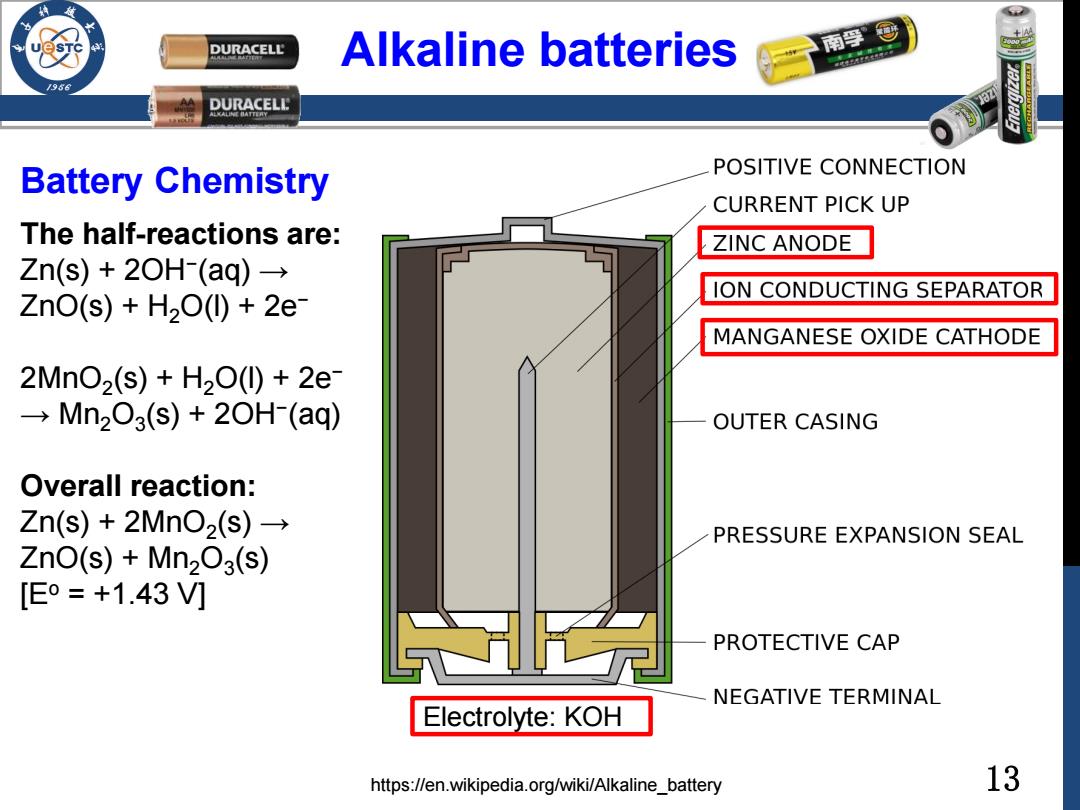
DURACELL Alkaline batteries 南停@ /98 DURACELL Battery Chemistry POSITIVE CONNECTION CURRENT PICK UP The half-reactions are: ZINC ANODE Zn(s)+2OH(aq)→ ION CONDUCTING SEPARATOR ZnO(s)+H200)+2e MANGANESE OXIDE CATHODE 2MnO2(s)+H200+2e →Mn203(s)+20H(aq) OUTER CASING Overall reaction: Zn(s)+2MnO2(s)→ PRESSURE EXPANSION SEAL ZnO(s)+Mn203(s) [Eo=+1.43V] PROTECTIVE CAP NEGATIVE TERMINAL Electrolyte:KOH https://en.wikipedia.org/wiki/Alkaline_battery 1313 Alkaline batteries The half-reactions are: Zn(s) + 2OH− (aq) → ZnO(s) + H2O(l) + 2e− 2MnO2 (s) + H2O(l) + 2e− → Mn2O3 (s) + 2OH− (aq) Overall reaction: Zn(s) + 2MnO2 (s) → ZnO(s) + Mn2O3 (s) [Eo = +1.43 V] https://en.wikipedia.org/wiki/Alkaline_battery Battery Chemistry Electrolyte: KOH