正在加载图片...
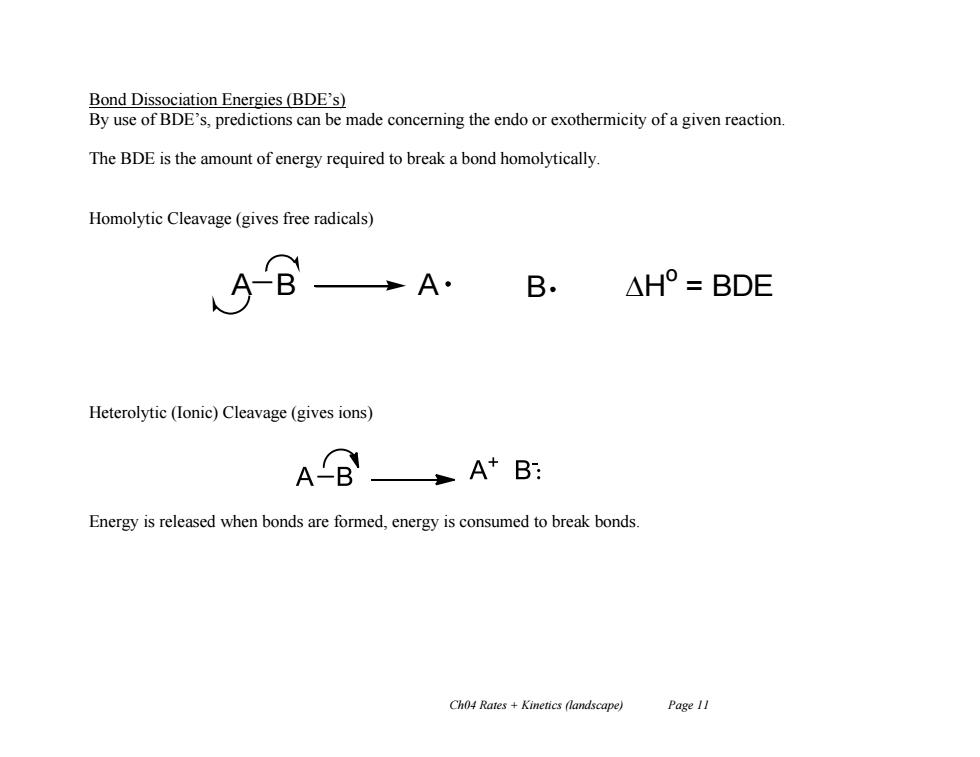
Bond Dissociation Energies(BDE's) By use of BDE's,predictions can be made concerning the endo or exothermicity of a given reaction. The BDE is the amount of energy required to break a bond homolytically. Homolytic Cleavage(gives free radicals) A· B △H°=BDE Heterolytic (Ionic)Cleavage(gives ions) AB A+B: Energy is released when bonds are formed,energy is consumed to break bonds. Ch04 Rates Kinetics (landscape) Page 11Ch04 Rates + Kinetics (landscape) Page 11 Bond Dissociation Energies (BDE’s) By use of BDE’s, predictions can be made concerning the endo or exothermicity of a given reaction. The BDE is the amount of energy required to break a bond homolytically. Homolytic Cleavage (gives free radicals) Heterolytic (Ionic) Cleavage (gives ions) Energy is released when bonds are formed, energy is consumed to break bonds. A B A B H o = BDE