正在加载图片...
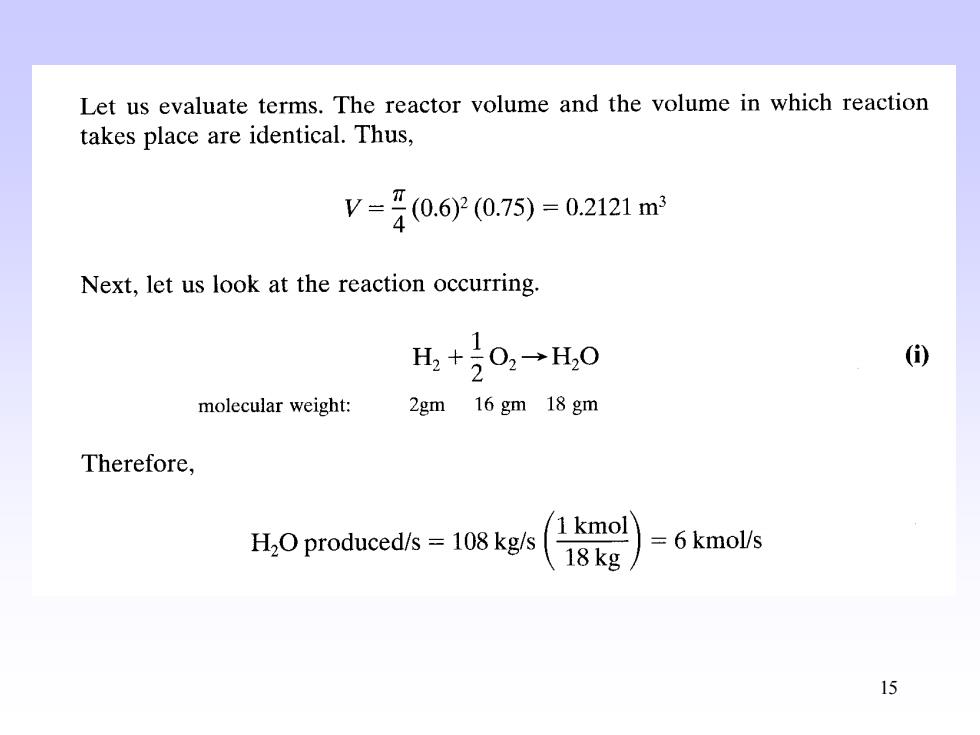
Let us evaluate terms.The reactor volume and the volume in which reaction takes place are identical.Thus, V=40.60.7)=02121m Next,let us look at the reaction occurring. 1 H,+20,→H,0 () molecular weight: 2gm 16 gm 18 gm Therefore, H2O produced/s =108 kg/s 1 kmol 18kg 6kmol/s 1515