
Chemical Reaction Engineering Teacher:Dr.&Prof.Kai Guo Beijing University of Chemical Technology
1 Chemical Reaction Engineering Teacher: Dr.&Prof. Kai Guo Beijing University of Chemical Technology
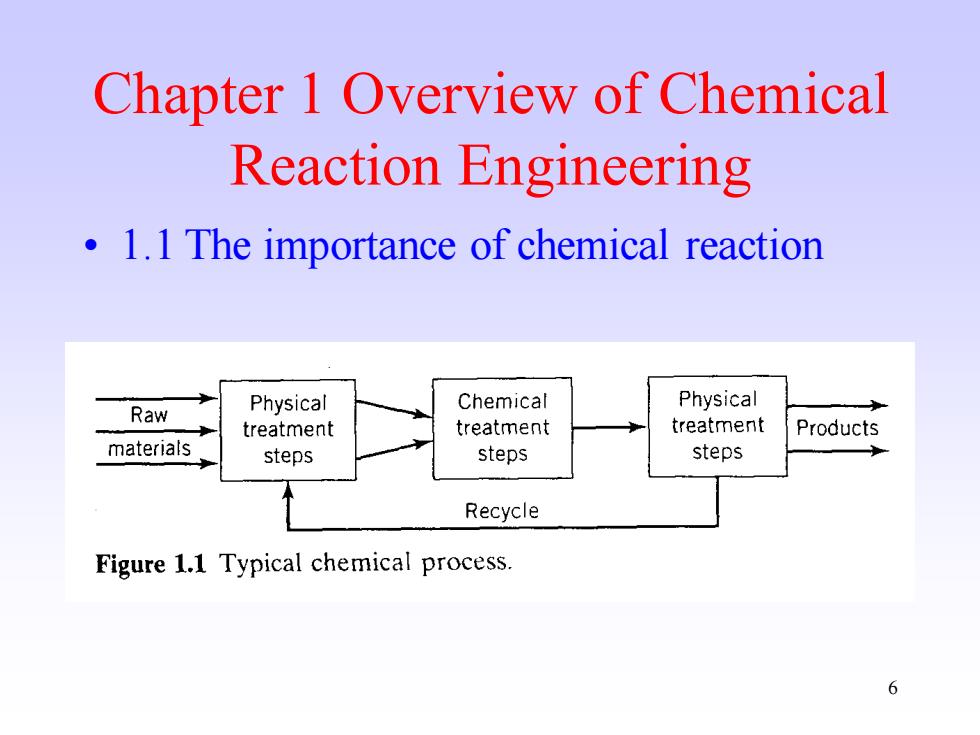
Chapter 1 Overview of Chemical Reaction Engineering 1.1 The importance of chemical reaction Chemical Physical Raw Physical treatment treatment treatment Products materials steps steps steps Recycle Figure 1.1 Typical chemical process. 6
6 Chapter 1 Overview of Chemical Reaction Engineering • 1.1 The importance of chemical reaction
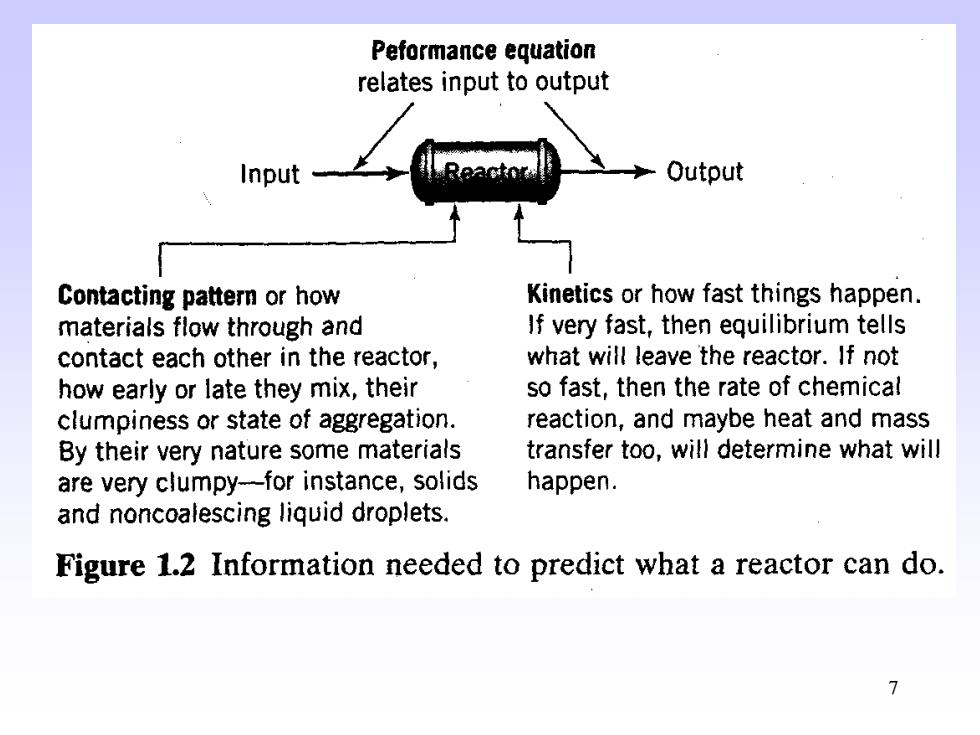
Peformance equation relates input to output Input- Output Contacting patter or how Kinetics or how fast things happen. materials flow through and If very fast,then equilibrium tells contact each other in the reactor, what will leave the reactor.If not how early or late they mix,their so fast,then the rate of chemical clumpiness or state of aggregation. reaction,and maybe heat and mass By their very nature some materials transfer too,will determine what will are very clumpy-for instance,solids happen. and noncoalescing liquid droplets. Figure 1.2 Information needed to predict what a reactor can do. 7
7
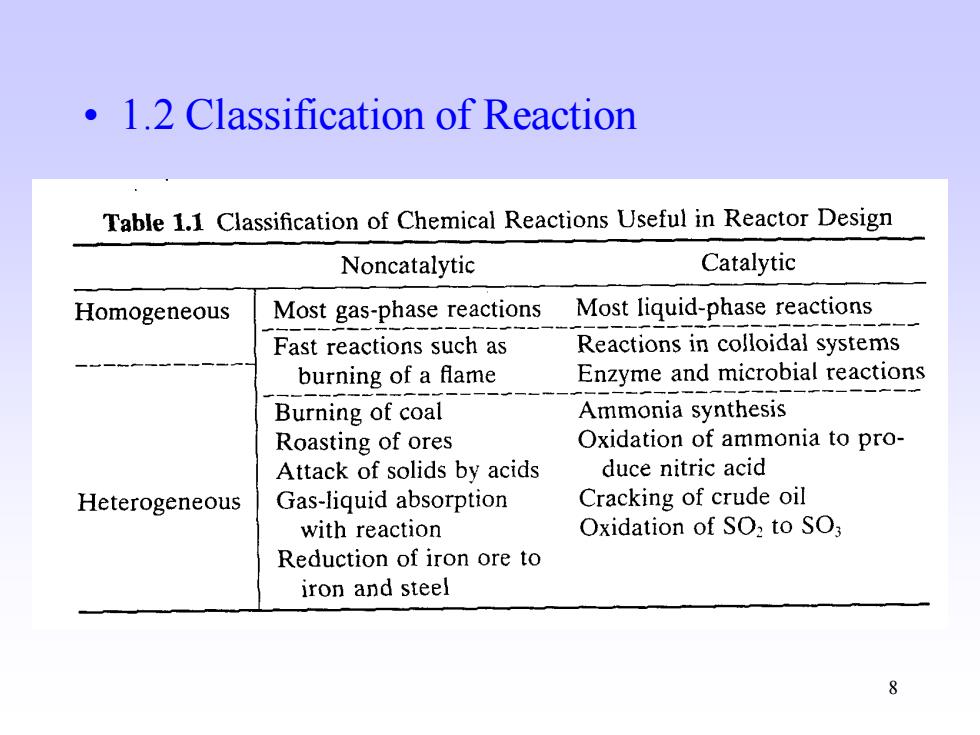
1.2 Classification of Reaction Table 1.1 Classification of Chemical Reactions Useful in Reactor Design Noncatalytic Catalytic Homogeneous Most gas-phase reactions Most liquid-phase reactions Fast reactions such as Reactions in colloidal systems burning of a flame Enzyme and microbial reactions Burning of coal Ammonia synthesis Roasting of ores Oxidation of ammonia to pro- Attack of solids by acids duce nitric acid Heterogeneous Gas-liquid absorption Cracking of crude oil with reaction Oxidation of SO2 to SO; Reduction of iron ore to iron and steel 8
8 • 1.2 Classification of Reaction
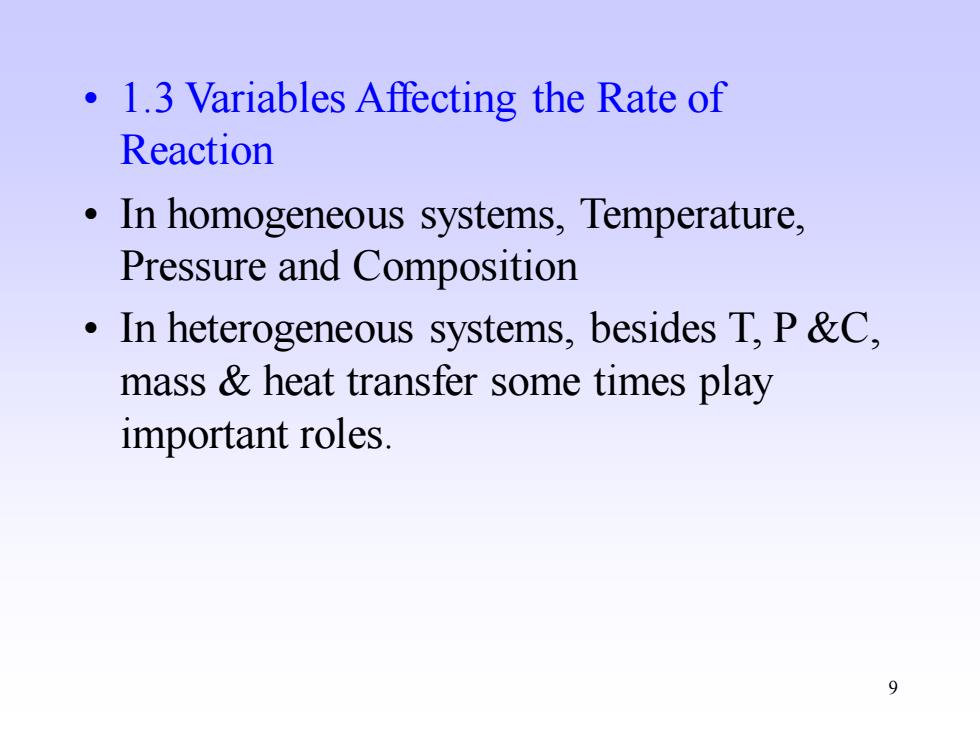
1.3 Variables Affecting the Rate of Reaction In homogeneous systems,Temperature, Pressure and Composition In heterogeneous systems,besides T,P &C, mass heat transfer some times play important roles 9
9 • 1.3 Variables Affecting the Rate of Reaction • In homogeneous systems, Temperature, Pressure and Composition • In heterogeneous systems, besides T, P &C, mass & heat transfer some times play important roles

1.4 Definition of Reaction Rate If the rate of change in number of moles of component i due to reaction is dN dt,the rate of reaction is defined as follows: Based on unit volum e of reacting fluid 1dN,_ mole i formed n=v dt (volume of fluid )(time) Based on unit mass of solid in fluid -solid system mole i formed w dt (mass of solid )(time) 10
10 • 1.4 Definition of Reaction Rate • If the rate of change in number of moles of component i due to reaction is dNi /dt, the rate of reaction is defined as follows: ( )( ) ( )( ) mass of solid i formed Based on unit mass of solid in fluid -solid system volume of fluid i formed Based on unit volum e of reacting fluid : time mole dt dN W 1 r time mole dt dN V 1 r i i i i = = = =
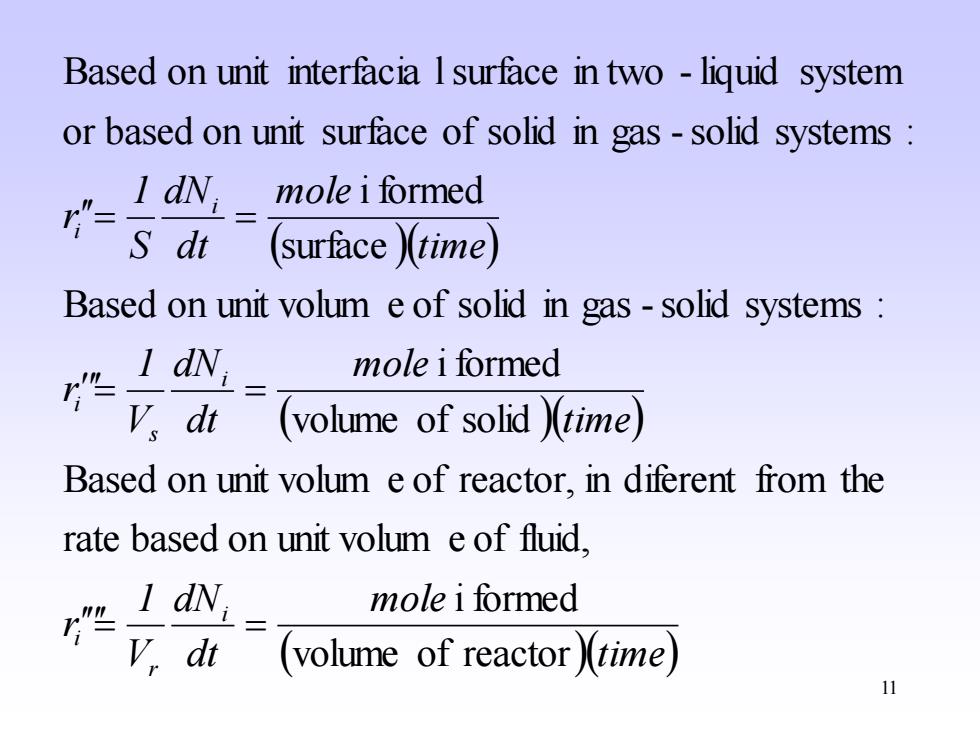
Based on unit interfacia I surface in two -liquid system or based on unit surface of solid in gas-solid systems I dN mole i formed (surface)(time) Based on unit volum e of solid in gas-solid systems I dNi mole i formed 'vdi (volume of solid )(time) Based on unit volum e of reactor,in diferent from the rate based on unit volum e of fluid, I dN; mole i formed V.dt (volume of reactor)(time) 11
11 ( )( ) ( )( ) ( )(time) mole dt dN V 1 r time mole dt dN V 1 r time mole dt dN S 1 r i r i i s i i i volume of reactor i formed rate based on unit volum e of fluid, Based on unit volum e of reactor, in diferent from the volume of solid i formed Based on unit volum e of solid in gas -solid systems : surface i formed or based on unit surface of solid in gas -solid systems : Based on unit interfacia lsurface in two - liquid system = = = = = =
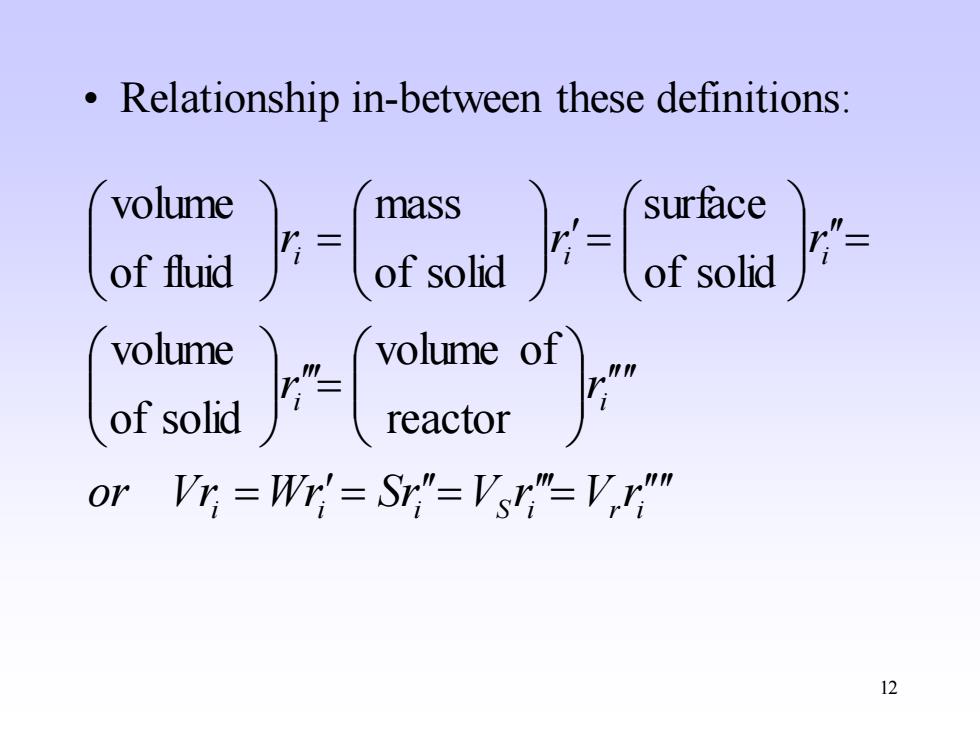
Relationship in-between these definitions: volume mass surface of fluid of solid volume volume of of solid reactor 0r V听=W=S"=V"=V" 12
12 • Relationship in-between these definitions: i i i S i r i i i i i i or Vr Wr Sr V r V r r r r r r = = = = = = = = reactor volume of of solid volume of solid surface of solid mass of fluid volume
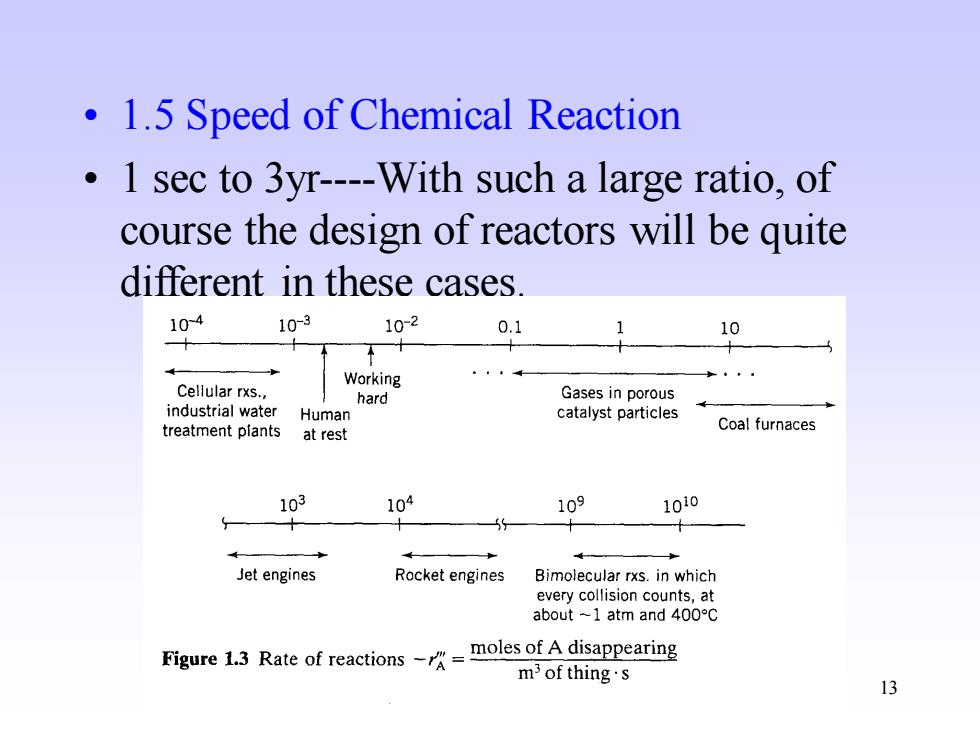
1.5 Speed of Chemical Reaction 1 sec to 3yr----With such a large ratio,of course the design of reactors will be quite different in these cases. 104 10-3 10-2 0.1 10 Working Cellular rxs., hard Gases in porous industrial water Human catalyst particles treatment plants Coal furnaces at rest 103 104 109 1010 X 5 Jet engines Rocket engines Bimolecular rxs.in which every collision counts,at about-1 atm and400°C Figure 1.3 Rate of reactions-moles ofAdisappearing m3 of thing's 13
13 • 1.5 Speed of Chemical Reaction • 1 sec to 3yr----With such a large ratio, of course the design of reactors will be quite different in these cases
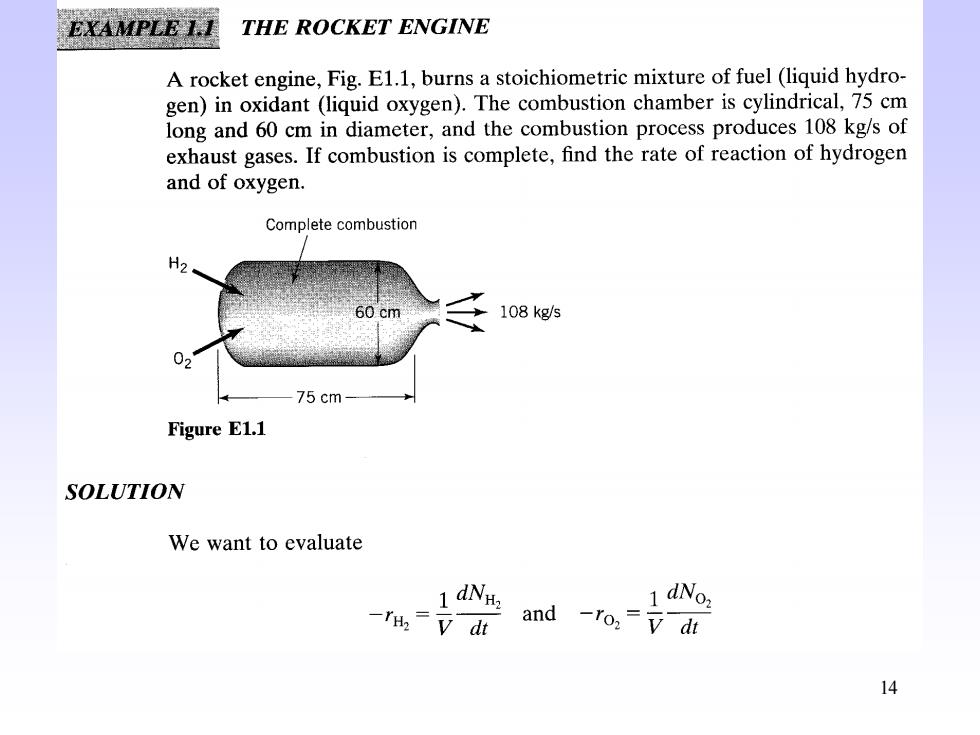
EXAMPLE 1.I THE ROCKET ENGINE A rocket engine,Fig.E1.1,burns a stoichiometric mixture of fuel (liquid hydro- gen)in oxidant (liquid oxygen).The combustion chamber is cylindrical,75 cm long and 60 cm in diameter,and the combustion process produces 108 kg/s of exhaust gases.If combustion is complete,find the rate of reaction of hydrogen and of oxygen. Complete combustion H2 60 cm 108 kg/s 02 75cm- Figure E1.1 SOLUTION We want to evaluate 1 dNH. -=v dt and -ro:=di 14
14