
Mam.Drugs2003,1,5-17 Marine Drugs ISSN1660-3397 www.mdpi.net/marinedrugs/ Review Drugs from the Sea-Opportunities and Obstacles Peter Proksch,*RuAngelie Edrada-Ebel and Rainer Ebel Institute of Pharmaceutical Biology,Heinrich-Heine-University Dusseldorf,Universitatsstr.1,Geb.26.23, D-40225D0 sseldorf,Germany.Tel.0049(211)-811-4163,Fax0049(211)-811-1923 *Author to whom correspondence should be addressed:E-mail:proksch@uni-duesseldorf.de Received:15 October 2003/Accepted:13 November 2003/Published:26 November 2003 Abstract:The supply problem with regard to drug development and sustainable production lies in the limited amounts of biomass of most marine invertebrates available from wild stocks.Thus,most pharmacologically active marine natural products can only be isolated in minute yields.Total synthesis of pharmacologically active natural products has been successfully established but is in many cases economically not feasible due to the complexity of the molecular structures and the low yields.To solve the pressing supply issue in marine drug discovery,other strategies appear to be more promising.One of these is mariculture which has successfully been established with the bryozoan Bugula neritina (the source of the bryostatins)and the tunicate Ecteinascidia turbinata(the source of ET-743).Another strategy involves partial synthesis from precursors which are biotechnologically available.An example is ET-743 that can be partially synthesized from safracin B which is a metabolite of Pseudomonas fluorescens.There have been many examples of striking structural similarities between natural products obtained from marine invertebrates and those of microbial origin which suggests that microorganisms living in their invertebrate hosts could be the actual producers of these secondary metabolites.With regard to sustainable biotechnological production of pharmacologically important metabolites from marine invertebrates and their
Mar. Drugs 2003, 1, 5-17 Marine Drugs ISSN 1660-3397 www.mdpi.net/marinedrugs/ Review Drugs from the Sea - Opportunities and Obstacles Peter Proksch,* RuAngelie Edrada-Ebel and Rainer Ebel Institute of Pharmaceutical Biology, Heinrich-Heine-University Düsseldorf, Universitätsstr. 1, Geb. 26.23, D-40225 Düsseldorf, Germany. Tel. 0049 (211)-811-4163, Fax 0049 (211)-811-1923 * Author to whom correspondence should be addressed; E-mail: proksch@uni-duesseldorf.de Received: 15 October 2003 / Accepted: 13 November 2003 / Published: 26 November 2003 Abstract: The supply problem with regard to drug development and sustainable production lies in the limited amounts of biomass of most marine invertebrates available from wild stocks. Thus, most pharmacologically active marine natural products can only be isolated in minute yields. Total synthesis of pharmacologically active natural products has been successfully established but is in many cases economically not feasible due to the complexity of the molecular structures and the low yields. To solve the pressing supply issue in marine drug discovery, other strategies appear to be more promising. One of these is mariculture which has successfully been established with the bryozoan Bugula neritina (the source of the bryostatins) and the tunicate Ecteinascidia turbinata (the source of ET-743). Another strategy involves partial synthesis from precursors which are biotechnologically available. An example is ET-743 that can be partially synthesized from safracin B which is a metabolite of Pseudomonas fluorescens. There have been many examples of striking structural similarities between natural products obtained from marine invertebrates and those of microbial origin which suggests that microorganisms living in their invertebrate hosts could be the actual producers of these secondary metabolites. With regard to sustainable biotechnological production of pharmacologically important metabolites from marine invertebrates and their

Mar.Drugs 2003,1 6 "endosymbionts",a more advanced strategy is to focus on cloning and expression of the respective key biosynthetic gene clusters.This molecular biological approach will open up new avenues for biotechnological production of drugs or drug candidates from the sea. Keywords:drugs from the sea,mariculture,endosymbionts,biotechnology Introduction Among the various sources for the development of new drugs,compounds from living organisms,so called natural products,are of particular significance [1].Approximately one third of today's best selling drugs are either natural products or have been developed based on lead structures provided by nature. Traditionally higher plants used to be the most prolific sources of drugs from nature.Recent examples of plant-derived anti-cancer drugs include paclitaxel (taxol)from Taxus brevifolia,etoposide (vepesid) derived by partial synthesis from the lignan podophyllotoxin isolated from Podophyllum peltatum and irinotecan(camptosar)obtained based on the lead structure of camptothecin isolated from Camptotheca acuminata.Use of medicinal plants is well documented throughout human history but is by no means restricted to humans as apes such a chimpanzees have been shown to use plants for the treatment of wounds or to fight intestinal parasites. Looking at drugs from nature it is surprising that up to now almost all medicinally used natural products or derivatives thereof were obtained from terrestrial organisms rather than from those inhabiting the sea.The oceans cover more than 70%of the earth'surface and are an indispensable source of protein for human nutrition.With regard to drug discovery and development,however,the oceans started to attract interest from pharmaceutical companies and research institutions only approximately 50 years ago with the discovery of the sponge-derived nucleosides spongothymidine and spongouridine [2](Fig.1). Since then Figure 1.Antiviral nucleosides from sponges spongothymidine spongouridine from the sponge Cryptotethia crypta
Mar. Drugs 2003, 1 6 “endosymbionts”, a more advanced strategy is to focus on cloning and expression of the respective key biosynthetic gene clusters. This molecular biological approach will open up new avenues for biotechnological production of drugs or drug candidates from the sea. Keywords: drugs from the sea, mariculture, endosymbionts, biotechnology. Introduction Among the various sources for the development of new drugs, compounds from living organisms, so called natural products, are of particular significance [1]. Approximately one third of today´s best selling drugs are either natural products or have been developed based on lead structures provided by nature. Traditionally higher plants used to be the most prolific sources of drugs from nature. Recent examples of plant-derived anti-cancer drugs include paclitaxel (taxol) from Taxus brevifolia, etoposide (vepesid) derived by partial synthesis from the lignan podophyllotoxin isolated from Podophyllum peltatum and irinotecan (camptosar) obtained based on the lead structure of camptothecin isolated from Camptotheca acuminata. Use of medicinal plants is well documented throughout human history but is by no means restricted to humans as apes such a chimpanzees have been shown to use plants for the treatment of wounds or to fight intestinal parasites. Looking at drugs from nature it is surprising that up to now almost all medicinally used natural products or derivatives thereof were obtained from terrestrial organisms rather than from those inhabiting the sea. The oceans cover more than 70% of the earth’surface and are an indispensable source of protein for human nutrition. With regard to drug discovery and development, however, the oceans started to attract interest from pharmaceutical companies and research institutions only approximately 50 years ago with the discovery of the sponge-derived nucleosides spongothymidine and spongouridine [2] (Fig. 1). Since then Figure 1. Antiviral nucleosides from sponges HN N HOH2C OH O OH O O CH3 HN N HOH2C OH O OH O O spongothymidine spongouridine from the sponge Cryptotethia crypta

Mar.Drugs 2003,1 7 well over 14,000 different natural products from marine organisms have been described [3],hundreds of patents describing new bioactive marine natural products have been filed [4]and approximately 10-15 different marine natural products are currently in clinical trials mostly in the areas of cancer,pain or inflammatory diseases [5].In spite of all efforts in marine natural products chemistry over the last three decades,no marine-derived compound(or analogue derived thereof)has been so far successfully launched to the drug market except for the anti-viral drug acyclovir and the antibiotic cephalosporin.Acyclovir which was synthetically known as Ara A,was modeled based on sponge-derived spongothymidine or spongouridine.Ara A along with its acetyl congener Ara U,were later isolated as natural products from the gorgonian Eunicella cavolini [6].The cephalosporins had first been described from fungi isolated close to a sewage effluent in the Mediterranean Sea.Thus,the major break through in drug discovery from the sea has so far not happened but is still eagerly awaited by those active in the field.What are plausible reasons and obstacles that have slowed down marine drug development and what could be promising research avenues that will possibly provide solutions for the future? The Supply Problem The fact that virtually no marine natural product can so far be found on the shelves of pharmacies is certainly not due to a limited chemical diversity of marine organisms.As stated above marine sources have provided well over 14,000 different natural products many of them being structurally unique and absent from terrestrial organisms.Incidence of biological or pharmacological activity in marine derived compounds is high especially with regard to cytotoxicity where marine-derived extracts clearly surpass those of terrestrial origin.It is no surprise therefore that marine natural products have their stronghold in the area of anti-cancer chemotherapy as indicated by the list of compounds currently under clinical investigation [5].Closer inspection of this list reveals that marine invertebrates such as sponges,tunicates bryozoans and molluscs are the most interesting sources of pharmacologically active metabolites whereas for example seaweeds that grow so abundantly or other marine algae appear to be less promising in comparison.The same holds true for marine vertebrates even though squalamine lactate(Fig.2)which is presently in clinical trials for the treatment of tumors has been derived from the cartilage of the dogfish shark,Squalus acanthias.A serious problem with regard to drug development and sustainable production that is shared by marine sponges,tunicates and other chemically interesting invertebrates lies in the patchy occurrence of these organisms and the limited amounts of biomass available from wild stocks.The supply problem that applies to many marine organisms and compounds derived from the latter has in the pas severely hampered drug development from marine sources. Many pharmacologically interesting marine natural products such as ecteinascidin 743(ET-743)(Fig 3)from the tunicate Ecteinascidia turbinata,the halichondrins from Lissodendoryx sponges(Fig.2),or the bryostatins from the bryozoan Bugula neritina(Fig.2)all of which are presently under clinical
Mar. Drugs 2003, 1 7 well over 14,000 different natural products from marine organisms have been described [3], hundreds of patents describing new bioactive marine natural products have been filed [4] and approximately 10 – 15 different marine natural products are currently in clinical trials mostly in the areas of cancer, pain or inflammatory diseases [5]. In spite of all efforts in marine natural products chemistry over the last three decades, no marine-derived compound (or analogue derived thereof) has been so far successfully launched to the drug market except for the anti-viral drug acyclovir and the antibiotic cephalosporin. Acyclovir which was synthetically known as Ara A, was modeled based on sponge-derived spongothymidine or spongouridine. Ara A along with its acetyl congener Ara U, were later isolated as natural products from the gorgonian Eunicella cavolini [6]. The cephalosporins had first been described from fungi isolated close to a sewage effluent in the Mediterranean Sea. Thus, the major break through in drug discovery from the sea has so far not happened but is still eagerly awaited by those active in the field. What are plausible reasons and obstacles that have slowed down marine drug development and what could be promising research avenues that will possibly provide solutions for the future? The Supply Problem The fact that virtually no marine natural product can so far be found on the shelves of pharmacies is certainly not due to a limited chemical diversity of marine organisms. As stated above marine sources have provided well over 14,000 different natural products many of them being structurally unique and absent from terrestrial organisms. Incidence of biological or pharmacological activity in marine derived compounds is high especially with regard to cytotoxicity where marine-derived extracts clearly surpass those of terrestrial origin. It is no surprise therefore that marine natural products have their stronghold in the area of anti-cancer chemotherapy as indicated by the list of compounds currently under clinical investigation [5]. Closer inspection of this list reveals that marine invertebrates such as sponges, tunicates, bryozoans and molluscs are the most interesting sources of pharmacologically active metabolites whereas for example seaweeds that grow so abundantly or other marine algae appear to be less promising in comparison. The same holds true for marine vertebrates even though squalamine lactate (Fig. 2) which is presently in clinical trials for the treatment of tumors has been derived from the cartilage of the dogfish shark, Squalus acanthias. A serious problem with regard to drug development and sustainable production that is shared by marine sponges, tunicates and other chemically interesting invertebrates lies in the patchy occurrence of these organisms and the limited amounts of biomass available from wild stocks. The supply problem that applies to many marine organisms and compounds derived from the latter has in the past severely hampered drug development from marine sources. Many pharmacologically interesting marine natural products such as ecteinascidin 743 (ET-743) (Fig. 3) from the tunicate Ecteinascidia turbinata, the halichondrins from Lissodendoryx sponges (Fig. 2), or the bryostatins from the bryozoan Bugula neritina (Fig. 2), all of which are presently under clinical
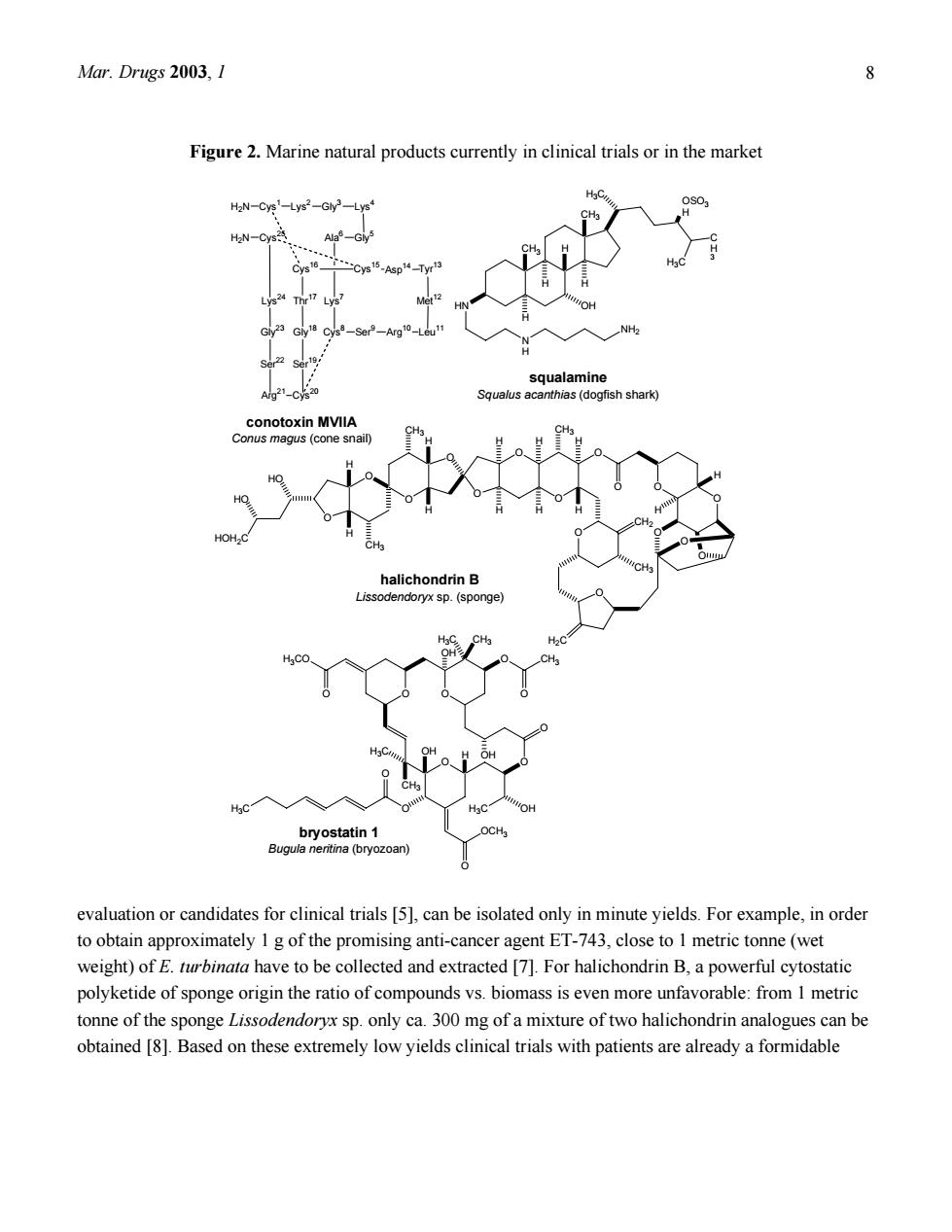
Mar.Drugs 2003,1 Figure 2.Marine natural products currently in clinical trials or in the market HaN-Cys-Lys2-Gly- Ser alamin 21-CV alus acanthas (dogfish shan so. evaluation or candidates for clinical trials [5],can be isolated only in minute yields.For example,in order to obtain approximately 1 g of the promising anti-cancer agent ET-743,close to 1 metric tonne(wet weight)of E.rbinata have to be collected and extracted []For halichondrin B,a powerful cytostatic polyketide of sponge origin the ratio of compounds vs.biomass is even more unfavorable:from I metric tonne of the sponge Lissodendoryx sp.only ca.300 mg of a mixture of two halichondrin analogues can be obtained [8].Based on these extremely low yields clinical trials with patients are already a formidable
Mar. Drugs 2003, 1 8 Figure 2. Marine natural products currently in clinical trials or in the market CH3 CH3 H H H H3C C H 3 H3C OSO3 H HN H N H NH2 OH O O O O O O O HO HO HOH2C H H CH3 CH3 H H H H O O O O O O O O O CH3 CH2 H2C H H H H CH3 H H O O O O O H3CO OH H3C CH3 O O CH3 OH O O H3C OH OH H CH3 H3C O OCH3 O H3C Cys1 Lys2 Gly3 Lys4 Gly5 Ala6 Lys7 Cys8 Ser 9 Arg10 Leu11 Met 12 Tyr 13 Asp14 Cys15 Cys16 Thr 17 Gly18 Ser 19 Cys20 Arg21 Ser 22 Gly23 Lys24 Cys25 H2N H2N halichondrin B Lissodendoryx sp. (sponge) bryostatin 1 Bugula neritina (bryozoan) squalamine Squalus acanthias (dogfish shark) conotoxin MVIIA Conus magus (cone snail) evaluation or candidates for clinical trials [5], can be isolated only in minute yields. For example, in order to obtain approximately 1 g of the promising anti-cancer agent ET-743, close to 1 metric tonne (wet weight) of E. turbinata have to be collected and extracted [7]. For halichondrin B, a powerful cytostatic polyketide of sponge origin the ratio of compounds vs. biomass is even more unfavorable: from 1 metric tonne of the sponge Lissodendoryx sp. only ca. 300 mg of a mixture of two halichondrin analogues can be obtained [8]. Based on these extremely low yields clinical trials with patients are already a formidable

Mar.Drugs 2003,1 9 challenge not to speak of the supply problem that will have to be met once a compound makes it to the market as a drug.For example,provided that the halichondrins will at one time be successfully launched for the treatment of cancer it is estimated that the annual need for these compounds will fall in the range of 1-5kg per year.This corresponds to roughly 3,000-16,000 metric tonnes of sponge biomass which would have to be collected and processed annually.It is clear that this task could never be completed successfully given the limited distribution of the respective sponges.Moreover destruction of the environment and extinction of species would be the inevitable consequences. Total synthesis of pharmacologically active marine natural products such as ET-743,halichondrin B. the bryostatins and others has been successfully established but is in many cases economically not feasible due to the complexity of the molecular structures and the low yields of final products achieved.A notable exception is the pain-killing peptide ntxin MVIIA (SNX-111)from the fish hunting marine mollusc Coms magus(generic name ziconotide)(Fig.2)which due to its peptide nature can be obtained in virtually unlimited amounts through synthesis [9]. Opportunities of Invertebrate Mariculture Other strategies to solve the pressing supply problem which is the key issue in marine drug discovery appear to be more promising in the foresecab futre.e of these approaches is mariculture which has been successfully developed in the past for example for fishes or shrimps and is already earning billions of dollars annually worldwide.Using mariculture for sustainable production of marine natural products other than polymeric carbohydrates such as agar,however,has only recently been established.Considerable progress in this area has been achieved with the bryozoan Bugula neritina(the source of the bryostatins) and with the tunicate E.turbinata (the source of ET-743)[7,10].Even though the biomass of marine invertebrates accessible through mariculture is up to now still not sufficient to meet the market demands provided that one of these compounds enters the drug market,this approach certainly holds considerable opportunities for the future and can be expected to contribute at least in some cases to a satisfactory solution of the supply problem.Nevertheless possible problems related to mass culture of marine invertebrates should not be forgotten since destruction of culture facilities and stocks by storms or by diseases are possible scenarios and will make predictability of the year to year harvest difficult. Arguments for a Microbial Origin of Bioactive Natural Products from Marine Invertebrates Close inspection of the structures of pharmacologically active natural products isolated from sponges tunicates and other marine invertebrates sometimes reveals striking chemical similarities to known microbial metabolites (Fig.3).For example,the cyclodepsipeptide jaspaklinolide (syn.jaspamide)that is isolated from marine Jaspis sponges and is used as a molecular tool in cell biology being a cell permeable
Mar. Drugs 2003, 1 9 challenge not to speak of the supply problem that will have to be met once a compound makes it to the market as a drug. For example, provided that the halichondrins will at one time be successfully launched for the treatment of cancer it is estimated that the annual need for these compounds will fall in the range of 1 – 5 kg per year. This corresponds to roughly 3,000 – 16,000 metric tonnes of sponge biomass which would have to be collected and processed annually. It is clear that this task could never be completed successfully given the limited distribution of the respective sponges. Moreover destruction of the environment and extinction of species would be the inevitable consequences. Total synthesis of pharmacologically active marine natural products such as ET-743, halichondrin B, the bryostatins and others has been successfully established but is in many cases economically not feasible due to the complexity of the molecular structures and the low yields of final products achieved. A notable exception is the pain-killing peptide ω-conotoxin MVIIA (SNX-111) from the fish hunting marine mollusc Conus magus (generic name ziconotide) (Fig. 2) which due to its peptide nature can be obtained in virtually unlimited amounts through synthesis [9]. Opportunities of Invertebrate Mariculture Other strategies to solve the pressing supply problem which is the key issue in marine drug discovery appear to be more promising in the foreseeable future. One of these approaches is mariculture which has been successfully developed in the past for example for fishes or shrimps and is already earning billions of dollars annually worldwide. Using mariculture for sustainable production of marine natural products other than polymeric carbohydrates such as agar, however, has only recently been established. Considerable progress in this area has been achieved with the bryozoan Bugula neritina (the source of the bryostatins) and with the tunicate E. turbinata (the source of ET-743) [7,10]. Even though the biomass of marine invertebrates accessible through mariculture is up to now still not sufficient to meet the market demands provided that one of these compounds enters the drug market, this approach certainly holds considerable opportunities for the future and can be expected to contribute at least in some cases to a satisfactory solution of the supply problem. Nevertheless possible problems related to mass culture of marine invertebrates should not be forgotten since destruction of culture facilities and stocks by storms or by diseases are possible scenarios and will make predictability of the year to year harvest difficult. Arguments for a Microbial Origin of Bioactive Natural Products from Marine Invertebrates Close inspection of the structures of pharmacologically active natural products isolated from sponges, tunicates and other marine invertebrates sometimes reveals striking chemical similarities to known microbial metabolites (Fig. 3). For example, the cyclodepsipeptide jaspaklinolide (syn. jaspamide) that is isolated from marine Jaspis sponges and is used as a molecular tool in cell biology being a cell permeable
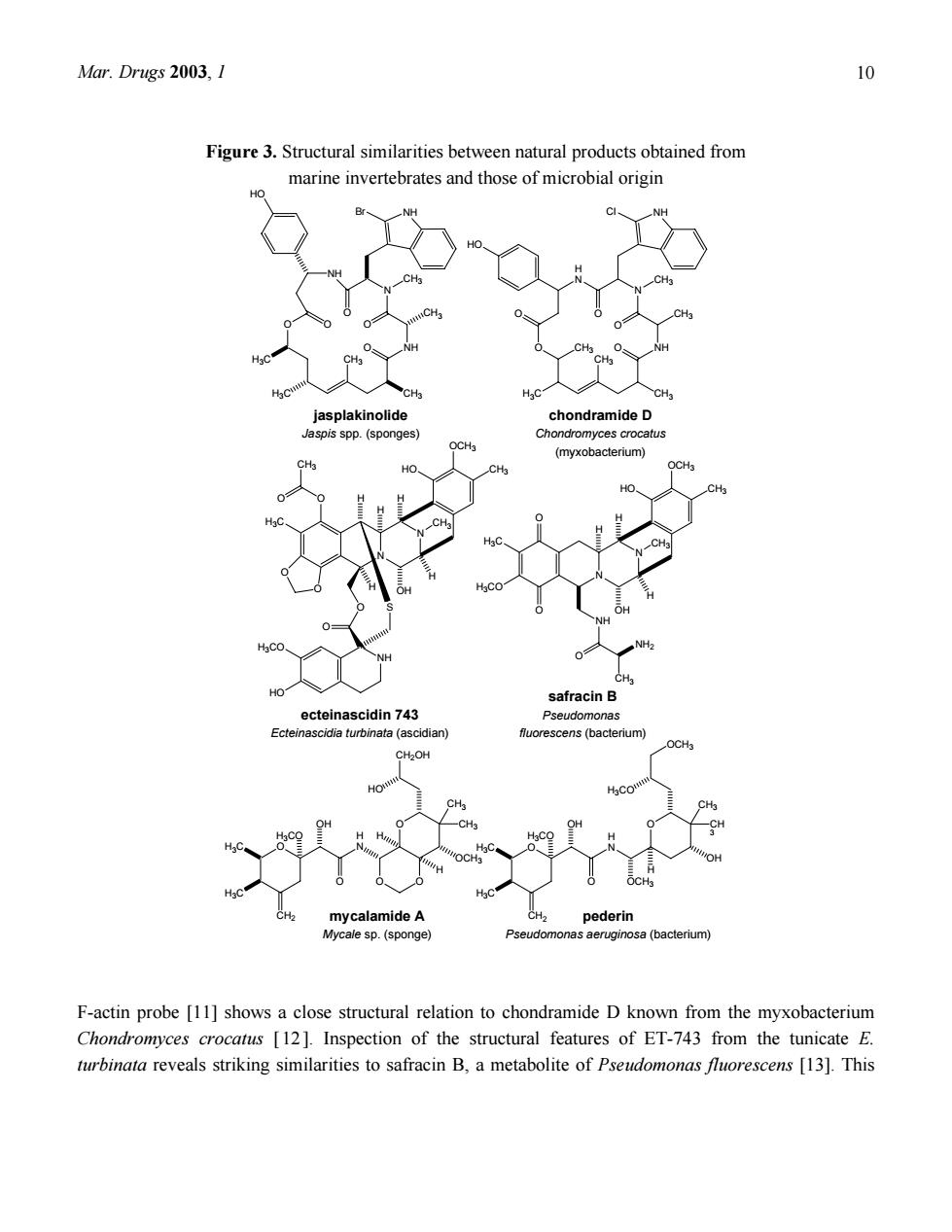
Mar.Drugs 2003,1 Figure 3.Structural similarities between natural products obtained from marine invertebrates and those of microbial origin ondramid mycalamide peder F-actin probe [11]shows a close structural relation to chondramide D known from the myxobacterium Chondromyces crocatus [12]Inspection of the structural features of ET-743 from the tunicate E. turbinata reveals striking similarities to safracin B,a metabolite of Pseudomonas fluorescens [13].This
Mar. Drugs 2003, 1 10 Figure 3. Structural similarities between natural products obtained from marine invertebrates and those of microbial origin CH3 CH3 N CH3 O NH O NH O O O NH Br H3C H3C HO CH3 CH3 CH3 N CH3 O NH O NH O O Cl H3C CH3 H N HO O CH3 N N OCH3 HO CH3 O O H3C O CH3 O H H H H H O NH HO H3CO S O OH CH3 N N OCH3 HO CH3 H H H OH CH3 O O H3CO H3C NH O NH2 CH3 O H N O OCH3 O C3 CH3 H3CO H OH OH H3CO H3C H3C CH2 OCH3 jasplakinolide Jaspis spp. (sponges) ecteinascidin 743 Ecteinascidia turbinata (ascidian) mycalamide A Mycale sp. (sponge) safracin B Pseudomonas fluorescens (bacterium) pederin Pseudomonas aeruginosa (bacterium) chondramide D Chondromyces crocatus (myxobacterium) O H N O O O O CH3 CH3 CH2OH HO H H OH OCH3 H3CO H3C H3C CH2 H F-actin probe [11] shows a close structural relation to chondramide D known from the myxobacterium Chondromyces crocatus [ 12]. Inspection of the structural features of ET-743 from the tunicate E. turbinata reveals striking similarities to safracin B, a metabolite of Pseudomonas fluorescens [13]. This

Mar.Drugs 2003,1 chemical similarity is in fact so pronounced that biotechnologically available cyanosafracin B provides a commercially feasible precursor for the partial synthesis of ET-743 [14].Symplostatin 1 (Fig.4).a close structural analogue of dolastatin 10 isolated from the marine mollusc Dolabella auricularia and currently in phase II clinical trials was found to be a metabolite of the blue-green alga Symploca hydnoides [15,16,17].Sometimes even identical metabolites are found both in microorganisms and marine invertebrates as exemplified by staurosporine recently isolated from the Micronesian tunicate Eudistom toealensis and the predatory flatworm Pseudoceros sp.[18].Staurosporine (Fig.4)is a typical metabolite of actinomycetes such as Saccharothrix aerocolonigenes subsp.staurorosporea.Derivatives of this alkaloid have received considerable attention due to their pronounced cytotoxic activity which at least in part results from inhibition of protein kinases The striking similarity of some microbial metabolites and natural products from marine invertebrates has aroused much speculation in the past(Fig.4).Given the fact that many marine invertebrates are filter feeders that ingest bacteria or other small particles from the inhaled sea water,it is possible that some bioactive metabolites previously isolated from sponges or tunicates are in fact of microbial origin and are actually taken up through the food chain.This has been shown for the protein phosphatase inhibitor okadaic acid obtained from Halichondria sponges.Later it was shown that this compound is produced by dinoflagellates of the genus Prorocentrum[19].It can be speculated that the food chain will also be responsible for other structurally unusual metabolites recovered from marine invertebrates even though each suspected case needs to be carefully examined and generalizations must be avoided.Sustainable production of the desired metabolites by biotechnological means could be envisaged if the respective producers are amenable to mass culturing and if a pharmacologically active natural product obtained from sponges or other invertebrates can be unequivocally traced to exogenous sources such as bacteria or cyanobacteria. However,sponges and other filter feeders do not only live from but also with bacteria as anatomical studies using electron microscopy usually reveal.Sometimes these associated bacteria are responsible fo a large proportion of the biomass of their host organism.For example,the bacterial concentration in the Mediterranean sponge Aplysina aerophoba exceeds that of the surrounding sea water by two or three orders of magnitude.Judging from microscopic analysis,up to 40%of the sponge volume consists of bacteria and cyanobacteria.For some marine sponges a direct contribution of associated bacteria (sometimes considered"endosymbionts")to natural product biosynthesis has been proven(Fig.5).The sponge Theonella swinhoei collected in the Philippines or in Micronesia contains the cyclic peptide theopalauamide and the macrolide swinholide A.Both compounds exhibit interesting biological activities Theopalauamide shows anti-fungal activity,swinholide A is strongly cytotoxic.Using differential centrifugation several cellular fractions were obtained from T.swinhoei.Theopalauamide was detected in filamentous bacteria whereas swinholide A was found in a fraction containing mostly unicellular bacteria [20.21].Based upon 16S rDNA gene sequencing the filaments that contained theopalauamide were shown
Mar. Drugs 2003, 1 11 chemical similarity is in fact so pronounced that biotechnologically available cyanosafracin B provides a commercially feasible precursor for the partial synthesis of ET-743 [14]. Symplostatin 1 (Fig. 4), a close structural analogue of dolastatin 10 isolated from the marine mollusc Dolabella auricularia and currently in phase II clinical trials was found to be a metabolite of the blue-green alga Symploca hydnoides [ 15 , 16 , 17 ]. Sometimes even identical metabolites are found both in microorganisms and marine invertebrates as exemplified by staurosporine recently isolated from the Micronesian tunicate Eudistoma toealensis and the predatory flatworm Pseudoceros sp. [18]. Staurosporine (Fig. 4) is a typical metabolite of actinomycetes such as Saccharothrix aerocolonigenes subsp. staurorosporea. Derivatives of this alkaloid have received considerable attention due to their pronounced cytotoxic activity which at least in part results from inhibition of protein kinases. The striking similarity of some microbial metabolites and natural products from marine invertebrates has aroused much speculation in the past (Fig. 4). Given the fact that many marine invertebrates are filter feeders that ingest bacteria or other small particles from the inhaled sea water, it is possible that some bioactive metabolites previously isolated from sponges or tunicates are in fact of microbial origin and are actually taken up through the food chain. This has been shown for the protein phosphatase inhibitor okadaic acid obtained from Halichondria sponges. Later it was shown that this compound is produced by dinoflagellates of the genus Prorocentrum [19]. It can be speculated that the food chain will also be responsible for other structurally unusual metabolites recovered from marine invertebrates even though each suspected case needs to be carefully examined and generalizations must be avoided. Sustainable production of the desired metabolites by biotechnological means could be envisaged if the respective producers are amenable to mass culturing and if a pharmacologically active natural product obtained from sponges or other invertebrates can be unequivocally traced to exogenous sources such as bacteria or cyanobacteria. However, sponges and other filter feeders do not only live from but also with bacteria as anatomical studies using electron microscopy usually reveal. Sometimes these associated bacteria are responsible for a large proportion of the biomass of their host organism. For example, the bacterial concentration in the Mediterranean sponge Aplysina aerophoba exceeds that of the surrounding sea water by two or three orders of magnitude. Judging from microscopic analysis, up to 40% of the sponge volume consists of bacteria and cyanobacteria. For some marine sponges a direct contribution of associated bacteria (sometimes considered “endosymbionts”) to natural product biosynthesis has been proven (Fig. 5). The sponge Theonella swinhoei collected in the Philippines or in Micronesia contains the cyclic peptide theopalauamide and the macrolide swinholide A. Both compounds exhibit interesting biological activities. Theopalauamide shows anti-fungal activity, swinholide A is strongly cytotoxic. Using differential centrifugation several cellular fractions were obtained from T. swinhoei. Theopalauamide was detected in filamentous bacteria whereas swinholide A was found in a fraction containing mostly unicellular bacteria [20,21]. Based upon 16S rDNA gene sequencing the filaments that contained theopalauamide were shown
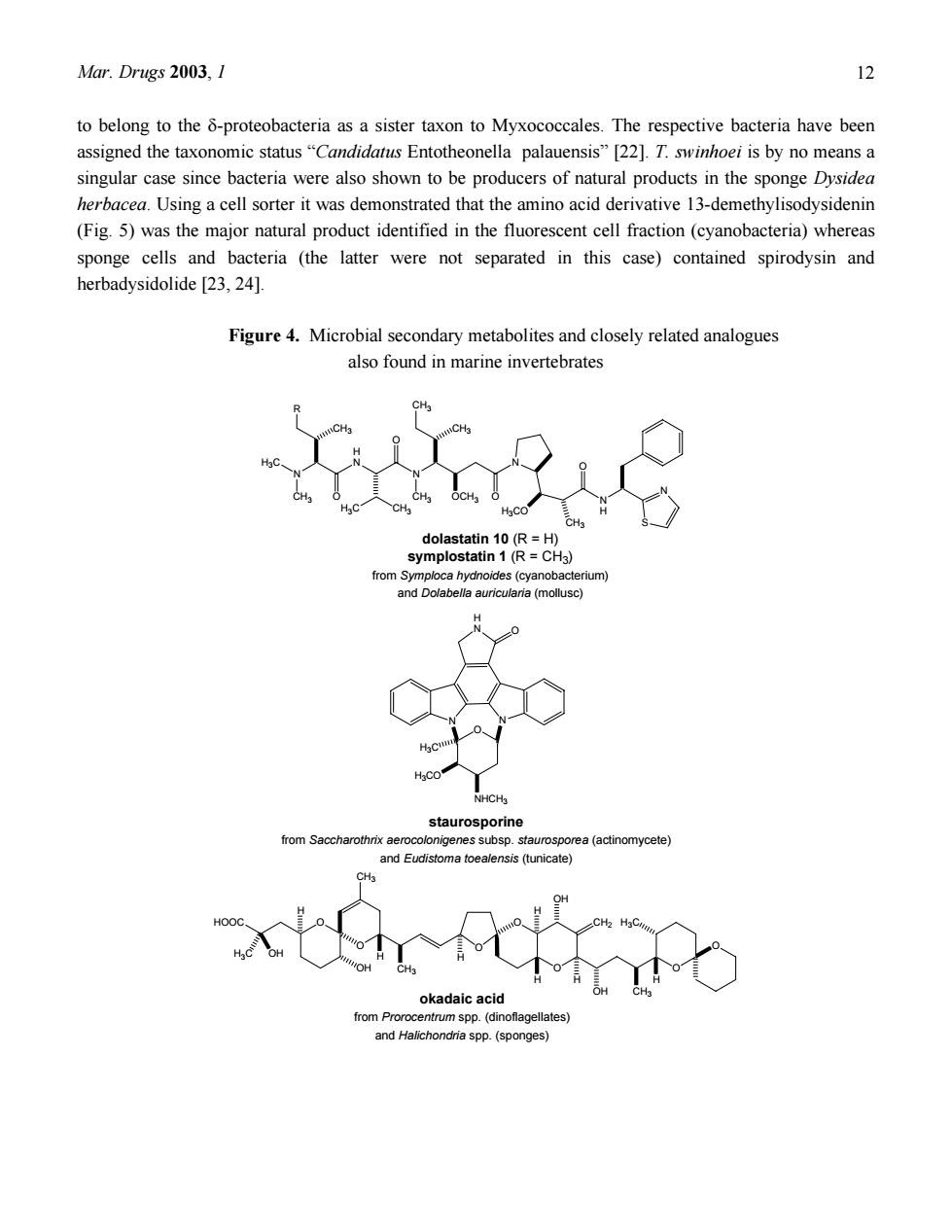
Mar.Drugs 2003,1 2 to belong to the 8-proteobacteria as a sister taxon to Myxococcales.The respective bacteria have been assigned the taxonomic status"Candidatus Entotheonella palauensis"[22].T.swinhoei is by no means a singular case since bacteria were also shown to be producers of natural products in the sponge Dysidea herbacea.Using a cell sorter it was demonstrated that the amino acid derivative 13-demethylisodysidenin (Fig.5)was the major natural product identified in the fluorescent cell fraction(cyanobacteria)whereas sponge cells and bacteria (the latter were not separated in this case)contained spirodysin and herbadysidolide [23,24]. Figure 4.Microbial secondary metabolites and closely related analogues also found in marine invertebrates rom Symploca hydnoides (cyanobacterium nd Eudistoma(tunicate) okadaic aci and Halichondria spp.(sponges)
Mar. Drugs 2003, 1 12 to belong to the δ-proteobacteria as a sister taxon to Myxococcales. The respective bacteria have been assigned the taxonomic status “Candidatus Entotheonella palauensis” [22]. T. swinhoei is by no means a singular case since bacteria were also shown to be producers of natural products in the sponge Dysidea herbacea. Using a cell sorter it was demonstrated that the amino acid derivative 13-demethylisodysidenin (Fig. 5) was the major natural product identified in the fluorescent cell fraction (cyanobacteria) whereas sponge cells and bacteria (the latter were not separated in this case) contained spirodysin and herbadysidolide [23, 24]. Figure 4. Microbial secondary metabolites and closely related analogues also found in marine invertebrates N O H N CH3 H3C CH3 H3C CH3 O N CH3 CH3 OCH3 O N H3CO CH3 O N H S N dolastatin 10 (R = H) symplostatin 1 (R = CH3) from Symploca hydnoides (cyanobacterium) and Dolabella auricularia (mollusc) CH3 N N H N O NHCH3 O H3C H3CO O O O O O O O CH3 H3C OH HOOC OH CH3 OH CH2 OH H3C CH3 H H H H H H H staurosporine from Saccharothrix aerocolonigenes subsp. staurosporea (actinomycete) and Eudistoma toealensis (tunicate) okadaic acid from Prorocentrum spp. (dinoflagellates) and Halichondria spp. (sponges) R

Mar.Drugs 2003,1 13 Can the putative microbial producers of bioactive invertebrate metabolites provide a solution to the supply problem? It would seem a logical step trying to isolate and cultivate putative bacterial producers outside of their invertebrate hosts in order to set up a sustainable and manageable source of pharmacologically active compounds.Attempts to do so have in fact been numerous.However,many (if not most)bacterial inhabitants in sponges appear to be highly selective with regard to culture media and conditions which probably reflects their evolutionary adaptation to the conditions provided by the host.Attempts to culture the theopalauamide producing bacteria from the sponge T.swinhoei,for example,have have failed so far. From the sponge 4.aerophoba less than 1%of the bacteria present within the host tissue could be cultured using the standard ZoBell medium [25].Thus,considerable effort will have to be devoted in the future into the design of novel growth media and culturing conditions for sponge-associated bacteria in order to make these highly interesting organisms culturable under in vitro conditions and to tap their unique biosynthetic capacity for a biotechnological production of pharmacologically active natural products.Should these efforts be ultimately successful,implications for solving the pressing supply problem for therapeutically relevant marine natural products would indeed seem tremendous. A further methodologically even more advanced approach with regard to a sustainable biotechnological production of pharmacologically important metabolites from marine invertebrates (including their "endosymbionts")will certainly focus on cloning and expression of the respective key biosynthetic gene clusters.Whereas this approach has so far not met with success for marine organisms the viability of this strategy was corroborated in a recent study on the genetic background of pederin production in Paederus beetles [26].Pederin is a polyketide amide present for example in Paederus fuscipes beetles and shows strong cytotoxicity against various human cancer cell lines invitro as well as invivo [27].Structurally pederin is closely related to mycalamide A(Fig.3)from the marine sponge Mycale sp.[28]that shows likewise high toxicity to eukaryotic cells 9].16 rRNA analysis of pederin containing beetles indicated the presence of a predominant symbiotic bacterium with a close relationship to Pseudomonas aeruginosa 30].The putative pederin biosynthesis genes were recently cloned from total DNA of P.fiscipes beetles [26].Sequence analysis of the gene cluster which includes a mixed modular polyketide synthase/nonribosomal peptide synthase and tailoring enzymes revealed typical bacterial gene architecture and homologies thus providing unequivocal proof for the prokaryotic origin of pederin.Based on the close structural homologies between pederin and mycalamide A it is more than likely that the latter compound will also originate from microorganisms rather than from sponge cells even though experimental proof for this hypothesis is not available so far. As shown for pederin,knowledge of the biosynthetic origin of the respective compound and of the key enzymes involved (polyketide synthase and nonribosomal peptide synthase)can be of considerable advantage in elucidating the role of endosymbionts for natural products in invertebrates.For many other
Mar. Drugs 2003, 1 13 Can the putative microbial producers of bioactive invertebrate metabolites provide a solution to the supply problem? It would seem a logical step trying to isolate and cultivate putative bacterial producers outside of their invertebrate hosts in order to set up a sustainable and manageable source of pharmacologically active compounds. Attempts to do so have in fact been numerous. However, many (if not most) bacterial inhabitants in sponges appear to be highly selective with regard to culture media and conditions which probably reflects their evolutionary adaptation to the conditions provided by the host. Attempts to culture the theopalauamide producing bacteria from the sponge T. swinhoei, for example, have have failed so far. From the sponge A. aerophoba less than 1% of the bacteria present within the host tissue could be cultured using the standard ZoBell medium [25]. Thus, considerable effort will have to be devoted in the future into the design of novel growth media and culturing conditions for sponge-associated bacteria in order to make these highly interesting organisms culturable under in vitro conditions and to tap their unique biosynthetic capacity for a biotechnological production of pharmacologically active natural products. Should these efforts be ultimately successful, implications for solving the pressing supply problem for therapeutically relevant marine natural products would indeed seem tremendous. A further methodologically even more advanced approach with regard to a sustainable biotechnological production of pharmacologically important metabolites from marine invertebrates (including their “endosymbionts”) will certainly focus on cloning and expression of the respective key biosynthetic gene clusters. Whereas this approach has so far not met with success for marine organisms the viability of this strategy was corroborated in a recent study on the genetic background of pederin production in Paederus beetles [26]. Pederin is a polyketide amide present for example in Paederus fuscipes beetles and shows strong cytotoxicity against various human cancer cell lines in vitro as well as in vivo [27]. Structurally, pederin is closely related to mycalamide A (Fig. 3) from the marine sponge Mycale sp. [28] that shows likewise high toxicity to eukaryotic cells [29]. 16S rRNA analysis of pederin containing beetles indicated the presence of a predominant symbiotic bacterium with a close relationship to Pseudomonas aeruginosa [30]. The putative pederin biosynthesis genes were recently cloned from total DNA of P. fuscipes beetles [26]. Sequence analysis of the gene cluster which includes a mixed modular polyketide synthase/nonribosomal peptide synthase and tailoring enzymes revealed typical bacterial gene architecture and homologies thus providing unequivocal proof for the prokaryotic origin of pederin. Based on the close structural homologies between pederin and mycalamide A it is more than likely that the latter compound will also originate from microorganisms rather than from sponge cells even though experimental proof for this hypothesis is not available so far. As shown for pederin, knowledge of the biosynthetic origin of the respective compound and of the key enzymes involved (polyketide synthase and nonribosomal peptide synthase) can be of considerable advantage in elucidating the role of endosymbionts for natural products in invertebrates. For many other

Mar.Drugs 2003,1 marine natural products,however,their biosynthetic origin is either completely unknown or only poorly understood [31,32].Obviously,advantages in this field will be important for understanding symbiotic relationships also of other eukaryotic hosts and their prokaryotic symbionts in the future. Figure 5.Localization of different metabolites in the sponge Theonella swinhoei tin fraction containin unicellular bacteria) 13-demethylisodysidenin spirodysin herbadysidolide (in fraction containing (infraction conainingsponge cells and bacteria)
Mar. Drugs 2003, 1 14 marine natural products, however, their biosynthetic origin is either completely unknown or only poorly understood [31, 32]. Obviously, advantages in this field will be important for understanding symbiotic relationships also of other eukaryotic hosts and their prokaryotic symbionts in the future. Figure 5. Localization of different metabolites in the sponge Theonella swinhoei HN O HN O NH NH NH NH Br CH3 O O CH2OHO OH NH HO O NH HN CH2OH NH HN O H3C OH O NH HO O HO O O O CH2OH HO N N O HO H3C O H2N H2N O OOC O H3C OCH3 CH3 OH H3C O O O CH3 OH CH3 O O OH OH H3C CH3 H3CO OH OH CH3 OH H3C O OCH3 CH3 CH3 HO CH3 O OCH3 H3C H H H3C CH3 O O H3C N CCl3 CCl3 HN O CH3 O CH3 N S H3C H H O H3COCO H3C CH3 spirodysin herbadysidolide (in fraction containing sponge cells and bacteria) 13-demethylisodysidenin (in fraction containing cyanobacteria) swinholide A (in fraction containing unicellular bacteria) theopalauamide (in fraction containing filamentous bacteria)