Chapter 17 Heterogeneous Reaction--Introduction Since more than one phase is present,the movement of material from phase to phase must be considered in the rate equation.Thus the rate expression in general will incorporate mass transfer terms in addition to the usual chemical kinetics term.These mass transfer terms are different in type and numbers in the different kinds of heterogeneous systems;hence,no single rate expression has general application. 1
1 Chapter 17 Heterogeneous Reaction--Introduction Since more than one phase is present,the movement of material from phase to phase must be considered in the rate equation. Thus the rate expression in general will incorporate mass transfer terms in addition to the usual chemical kinetics term. These mass transfer terms are different in type and numbers in the different kinds of heterogeneous systems; hence, no single rate expression has general application
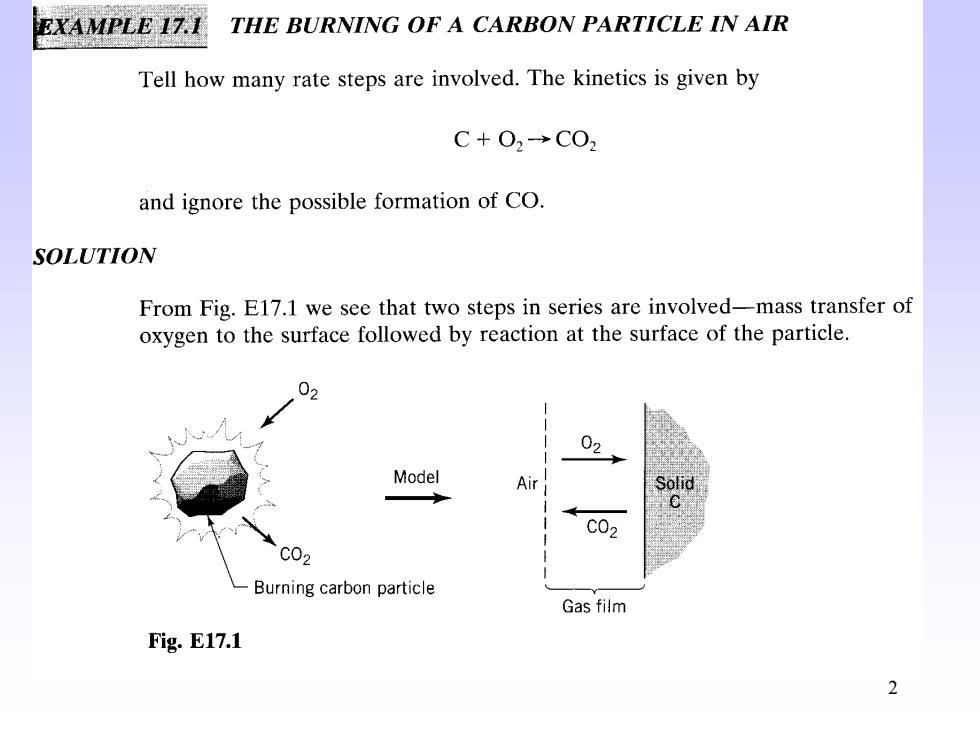
EXAMPLE IZ.I THE BURNING OF A CARBON PARTICLE IN AIR Tell how many rate steps are involved.The kinetics is given by C+O2→CO2 and ignore the possible formation of CO. SOLUTION From Fig.E17.1 we see that two steps in series are involved-mass transfer of oxygen to the surface followed by reaction at the surface of the particle. 03 02 Model Air C02 c02 Burning carbon particle Gas film Fig.E17.1 2
2
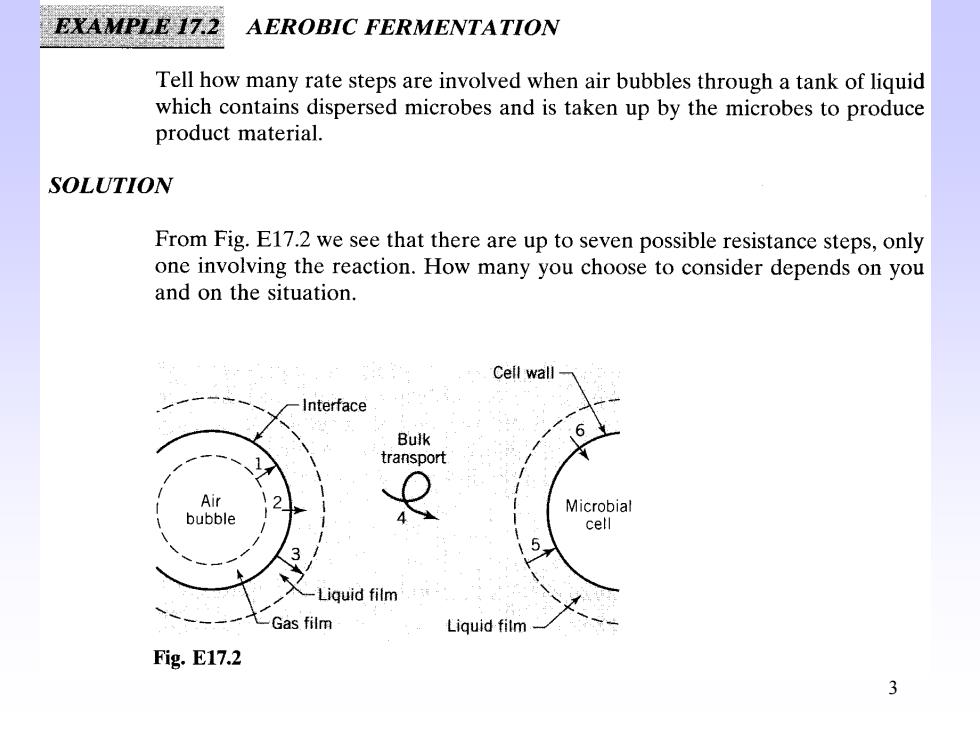
EXAMPLE 17.2 AEROBIC FERMENTATION Tell how many rate steps are involved when air bubbles through a tank of liquid which contains dispersed microbes and is taken up by the microbes to produce product material. SOLUTION From Fig.E17.2 we see that there are up to seven possible resistance steps,only one involving the reaction.How many you choose to consider depends on you and on the situation. Cell wall Interface Bulk transport Air Microbial bubble 4 cell 5, 3 Liquid film Gas film Liquid film- Fig.E17.2 3
3
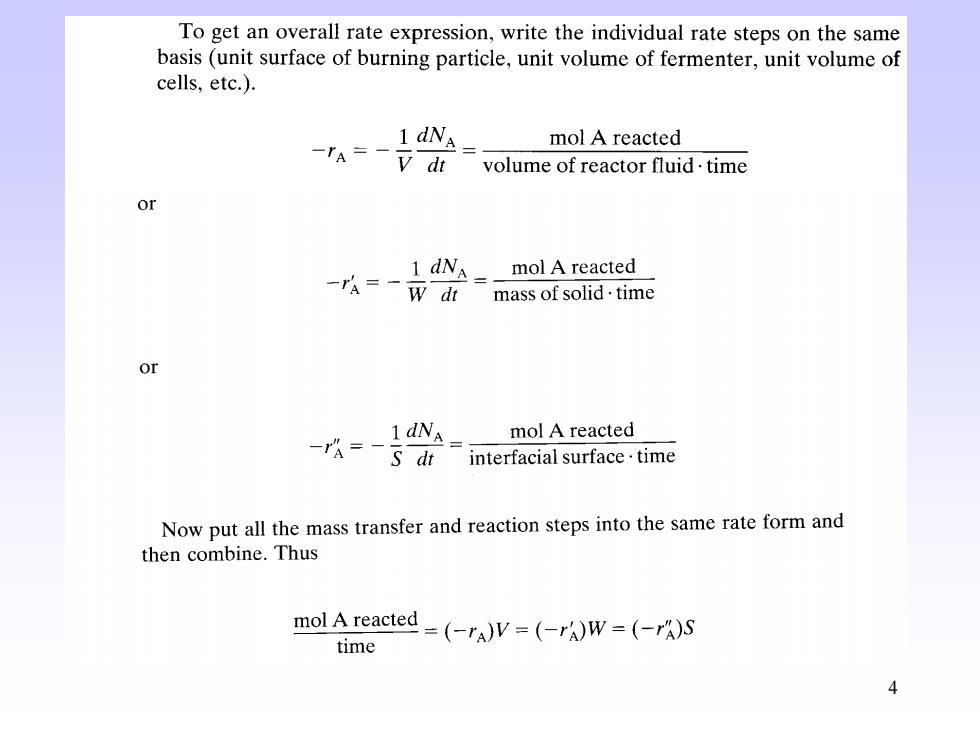
To get an overall rate expression,write the individual rate steps on the same basis(unit surface of burning particle,unit volume of fermenter,unit volume of cells,etc.). 1 dNA= -rA=-V dt mol A reacted volume of reactor fluid.time or 1 dN mol A reacted 一ra=一wdt mass of solid.time or 1dNA= -=-s di mol A reacted interfacial surface.time Now put all the mass transfer and reaction steps into the same rate form and then combine.Thus mol Areacted-(-rA)V=(rAw-(A)S time 4
4

or V and if the steps are in series,as in Examples 17.1 and 17.2 roverall =r1=r2=r3 If they are in parallel roverall=r1 +r2 Consider steps in series.In general,if all the steps are linear in concentration, then it is easy to combine them.However,if any of the steps are nonlinear,then you will get a messy overall expression.Therefore,you may try to bypass this nonlinear step in one of various ways.Approximating the ra versus Ca curve by a first-order expression is probably the most useful procedure. Another point:in combining rates we normally do not know the concentration of materials at intermediate conditions,so these are the concentrations that we eliminate in combining rates.Example 17.3 shows this. 5
5
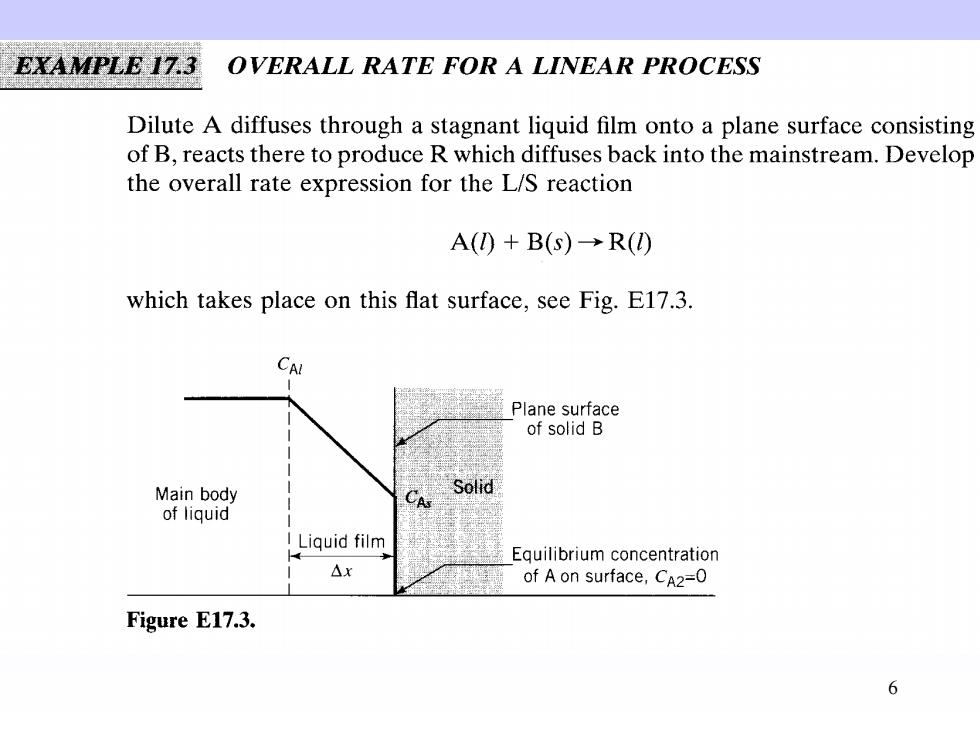
EXAMPLE 17.3 OVERALL RATE FOR A LINEAR PROCESS Dilute A diffuses through a stagnant liquid film onto a plane surface consisting of B,reacts there to produce R which diffuses back into the mainstream.Develop the overall rate expression for the L/S reaction A()+B(S)→R() which takes place on this flat surface,see Fig.E17.3. CAL Plane surface of solid B Main body Solid A of liquid I Liquid film Equilibrium concentration △x of A on surface,CA2=0 Figure E17.3. 6
6
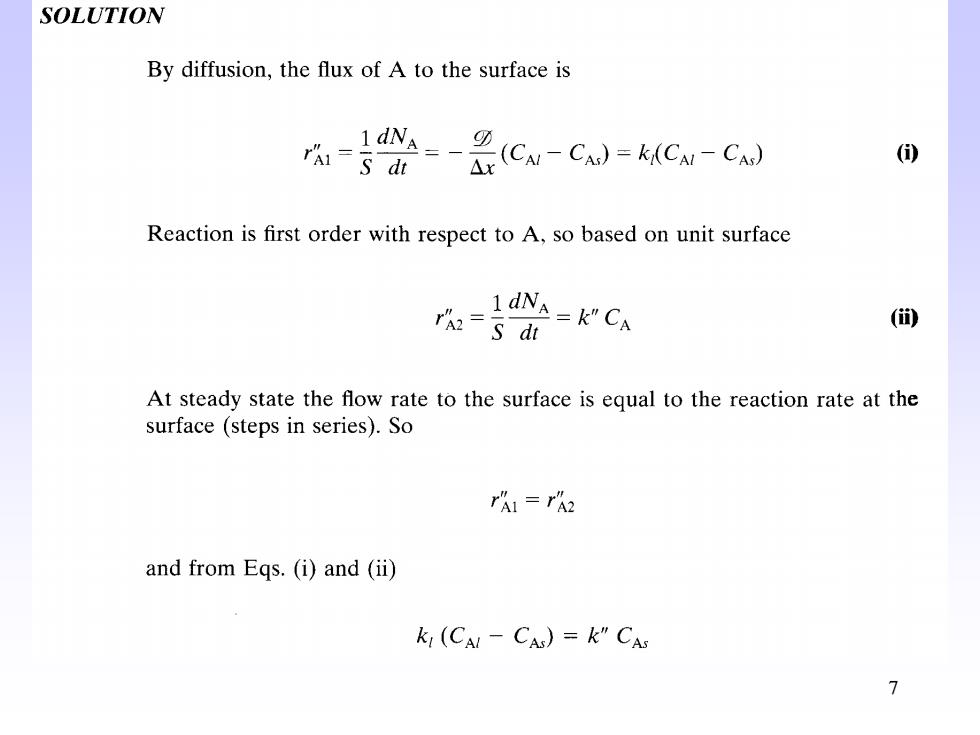
SOLUTION By diffusion,the flux of A to the surface is 4-a0=-是cw-c小=C-C rm=s di () Reaction is first order with respect to A,so based on unit surface 1dNA=k"CA rhe=S di (的 At steady state the flow rate to the surface is equal to the reaction rate at the surface (steps in series).So rAI=TA2 and from Eqs.(i)and (ii) k,(CA-CA)=k”CAs 7
7
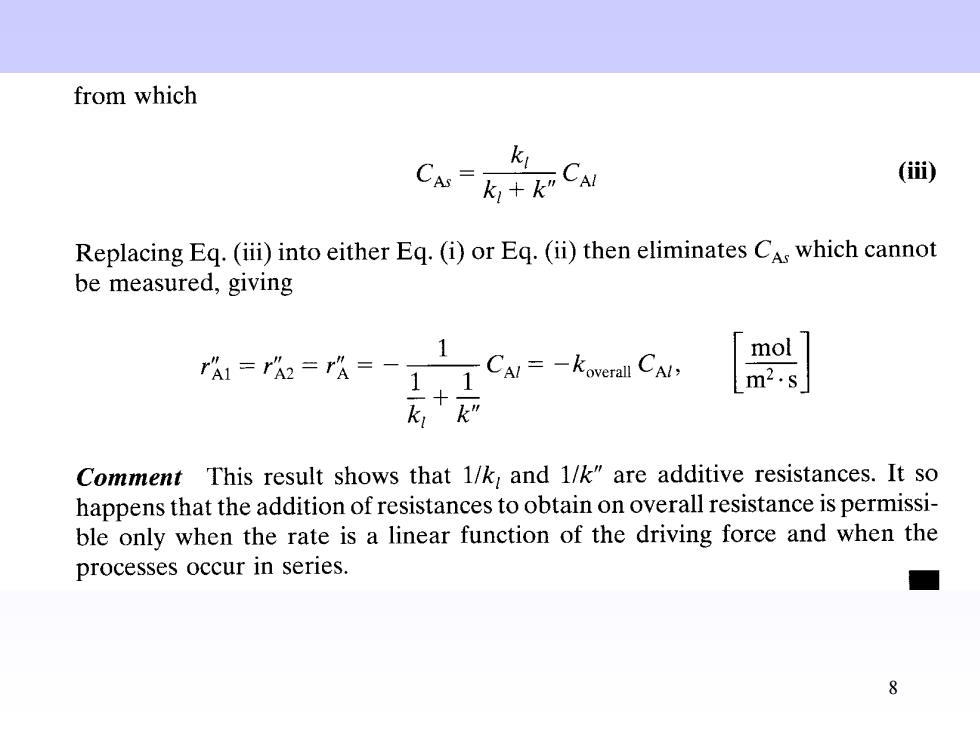
from which k CCM () Replacing Eq.(iii)into either Eq.(i)or Eq.(ii)then eliminates CAs which cannot be measured,giving 1 mol rA1=r=rA=-1 -CAl=-koverall CAl, m2.s k,k” Comment This result shows that 1/k and 1/k"are additive resistances.It so happens that the addition of resistances to obtain on overall resistance is permissi- ble only when the rate is a linear function of the driving force and when the processes occur in series. 8
8
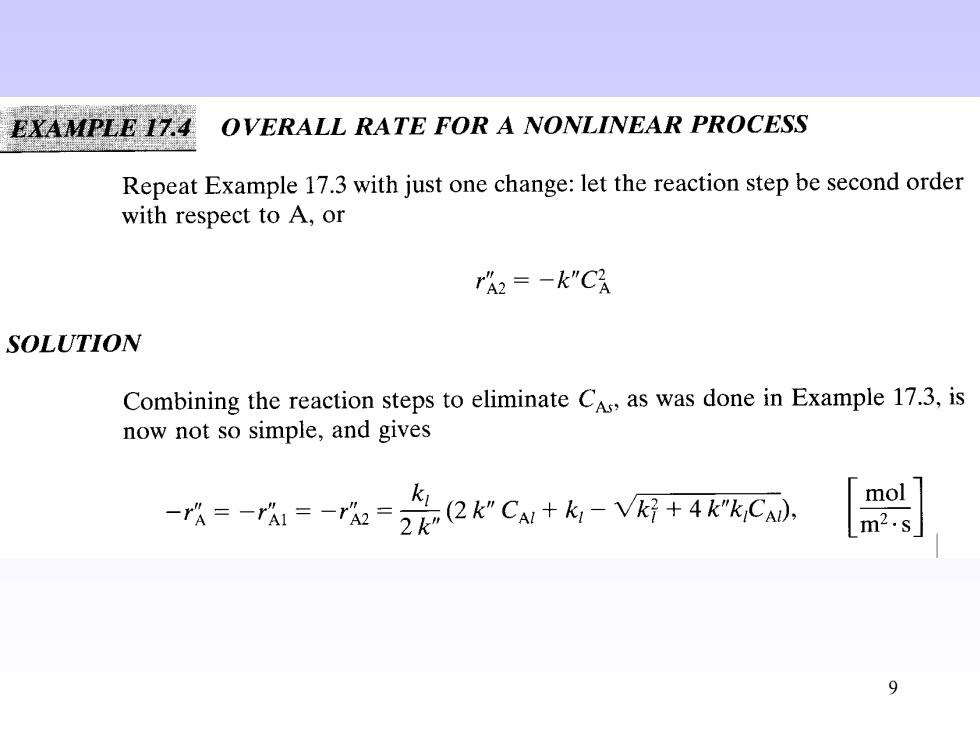
E17.4 OVERALL RATE FOR A NONLINEAR PROCESS Repeat Example 17.3 with just one change:let the reaction step be second order with respect to A,or rh2=-k"Ch SOLUTION Combining the reaction steps to eliminate CAs,as was done in Example 17.3,is now not so simple,and gives -店=-r=元=会2rC+-网+xC, m2.s 9
9
Contacting Patterns for Two-Phase Systems There are many ways that two phases can be contacted,and for each the design equation will be unique. When real flow deviates considerably from ideal flow patterns,we can do one of the two things:we may develop models to mirror actual flow closely,or we may calculate performance with ideal patterns which bracket actual flow. Five flow patterns: 10
10 • Contacting Patterns for Two-Phase Systems There are many ways that two phases can be contacted,and for each the design equation will be unique. • When real flow deviates considerably from ideal flow patterns, we can do one of the two things: we may develop models to mirror actual flow closely, or we may calculate performance with ideal patterns which bracket actual flow. • Five flow patterns: