正在加载图片...
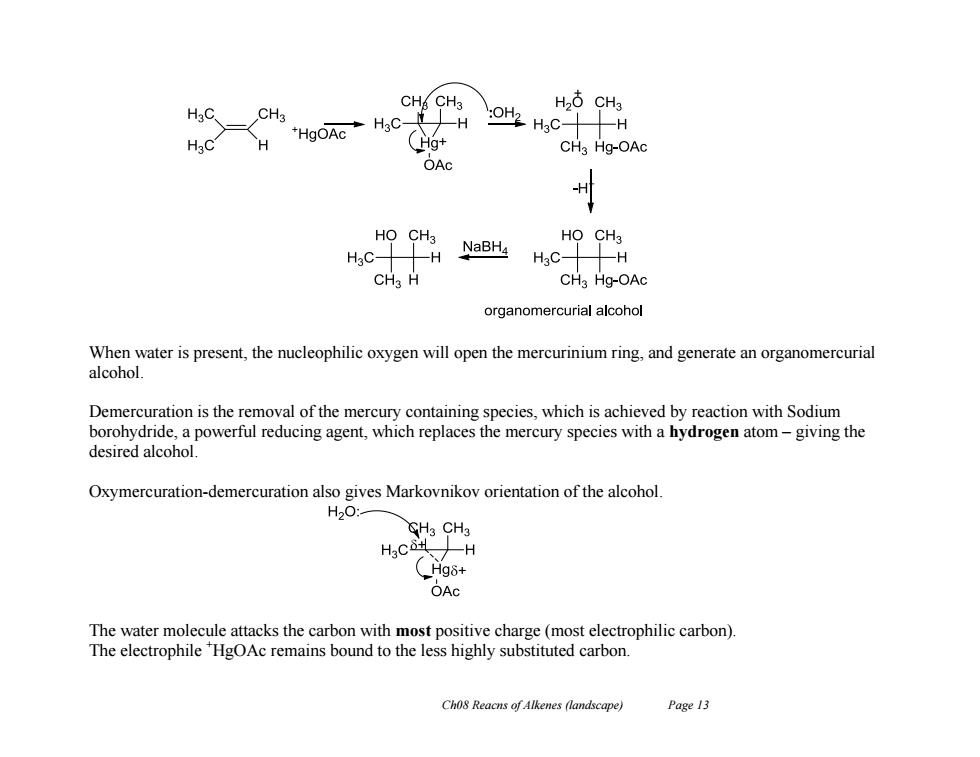
CH CH3 H26 CH3 HgC. CH3 o4Hc十H HaC H CH3 Hg-OAc OAc H HO CH3 HsC-H NaBHa HO CH3 H3C- H CHa H CHa Hg-OAc organomercurial alcohol When water is present,the nucleophilic oxygen will open the mercurinium ring,and generate an organomercurial alcohol. Demercuration is the removal of the mercury containing species,which is achieved by reaction with Sodium borohydride,a powerful reducing agent,which replaces the mercury species with a hydrogen atom-giving the desired alcohol. Oxymercuration-demercuration also gives Markovnikov orientation of the alcohol. H2O: CH3CH3 、 -H _Hg8+ OAc The water molecule attacks the carbon with most positive charge(most electrophilic carbon) The electrophile HgOAc remains bound to the less highly substituted carbon. Cho8 Reacns of Alkenes (landscape) Page 13Ch08 Reacns of Alkenes (landscape) Page 13 When water is present, the nucleophilic oxygen will open the mercurinium ring, and generate an organomercurial alcohol. Demercuration is the removal of the mercury containing species, which is achieved by reaction with Sodium borohydride, a powerful reducing agent, which replaces the mercury species with a hydrogen atom – giving the desired alcohol. Oxymercuration-demercuration also gives Markovnikov orientation of the alcohol. The water molecule attacks the carbon with most positive charge (most electrophilic carbon). The electrophile +HgOAc remains bound to the less highly substituted carbon