正在加载图片...
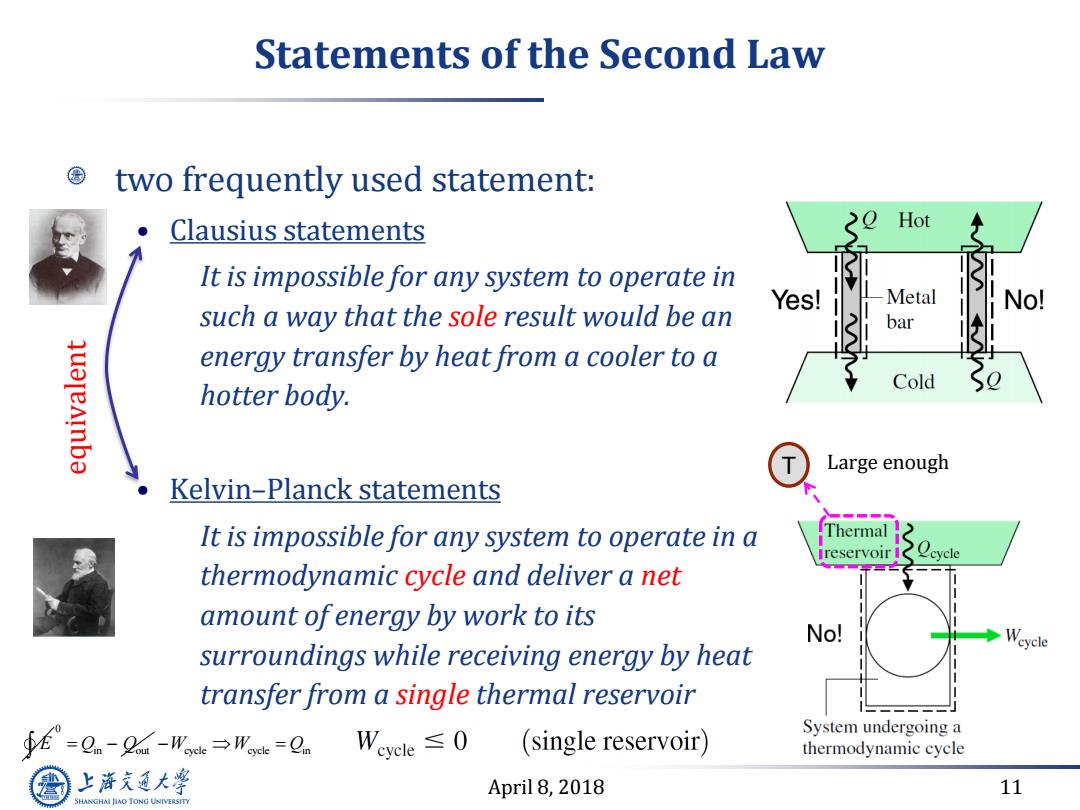
Statements of the Second Law two frequently used statement: Clausius statements >O Hot It is impossible for any system to operate in Yes! Metal No! such a way that the sole result would be an bar qualeainba energy transfer by heat from a cooler to a Cold hotter body. 入 Large enough Kelvin-Planck statements It is impossible for any system to operate in a Thermal reservoir eycle thermodynamic cycle and deliver a net amount ofenergy by work to its No! surroundings while receiving energy by heat transfer from a single thermal reservoir ∮f°=Qn-2-W→用k=Q。Weycle≤0 System undergoing a (single reservoir) thermodynamic cycle 上游充通大 April 8,2018 11 SHANGHAI JLAO TONG UNIVERSITYApril 8, 2018 11 Statements of the Second Law two frequently used statement: • Clausius statements It is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter body. • Kelvin–Planck statements It is impossible for any system to operate in a thermodynamic cycle and deliver a net amount of energy by work to its surroundings while receiving energy by heat transfer from a single thermal reservoir equivalent T Large enough E 0 Q Q in out W W Q cycle cycle in