正在加载图片...
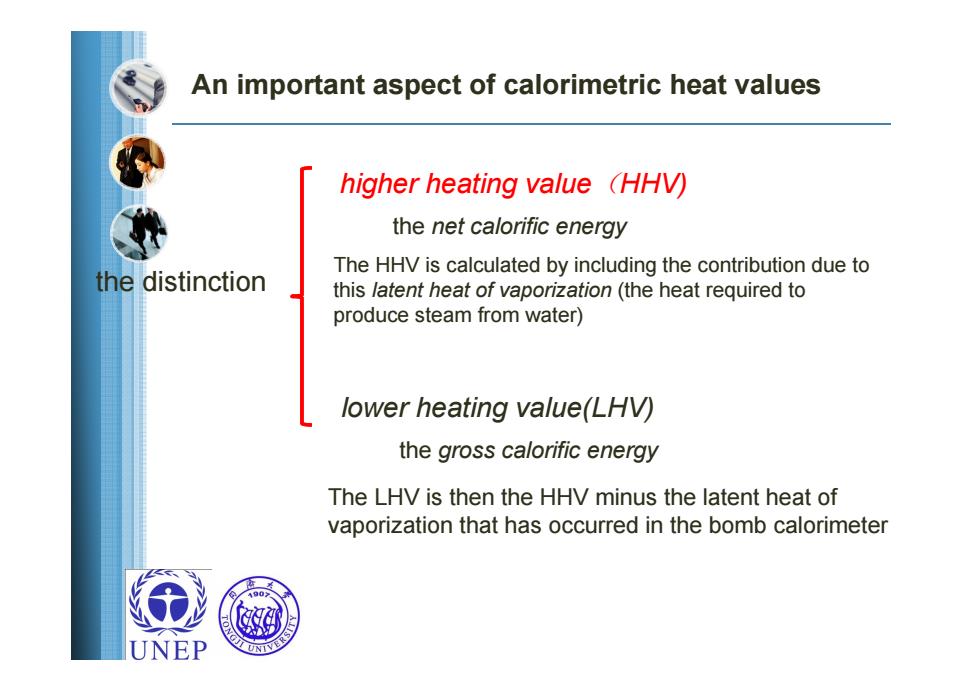
An important aspect of calorimetric heat values higher heating value (HHV) the net calorific energy The HHV is calculated by including the contribution due to the distinction this latent heat of vaporization(the heat required to produce steam from water) lower heating value(LHV) the gross calorific energy The LHV is then the HHV minus the latent heat of vaporization that has occurred in the bomb calorimeter UNEPthe gross calorific energy the net calorific energy the distinction higher heating value(HHV) lower heating value(LHV) The HHV is calculated by including the contribution due to this latent heat of vaporization (the heat required to produce steam from water) The LHV is then the HHV minus the latent heat of vaporization that has occurred in the bomb calorimeter An important aspect of calorimetric heat values