
UNEP Environmental Science--- Solid Waste Management(3)Incineration Niu Dongjie niudongjie@tongji.edu.cn
Environmental Science--- Solid Waste Management (3) Incineration Niu Dongjie niudongjie@tongji.edu.cn

Table of content o Heat Value of Refuse o Materials and Thermal Balance o Combustion Hardware Used for MSW o Undesirable Effects of Combustion UNEP
Table of content Heat Value of Refuse Materials and Thermal Balance Combustion Hardware Used for MSW Undesirable Effects of Combustion
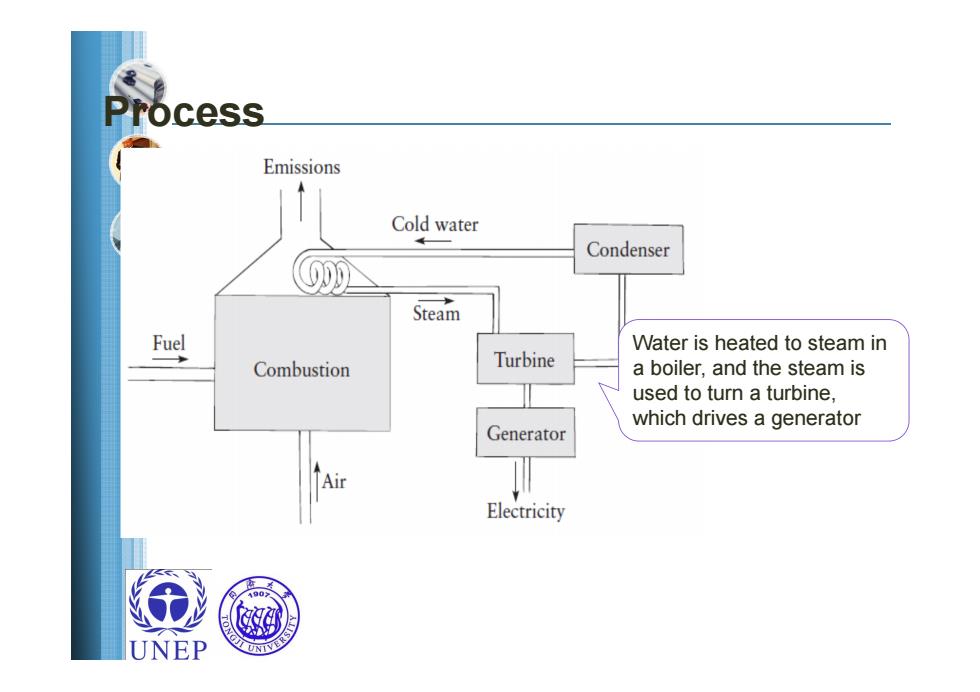
cess Emissions Cold water Condenser Steam Fuel Water is heated to steam in Combustion Turbine a boiler,and the steam is used to turn a turbine, which drives a generator Generator Air Electricity UNEP
Process Water is heated to steam in a boiler, and the steam is used to turn a turbine, which drives a generator
Heat Value of Refuse ●ultimate analysis compositional analysis o proximate analysis ●calorimetry UNEP
Heat Value of Refuse ultimate analysis compositional analysis proximate analysis calorimetry
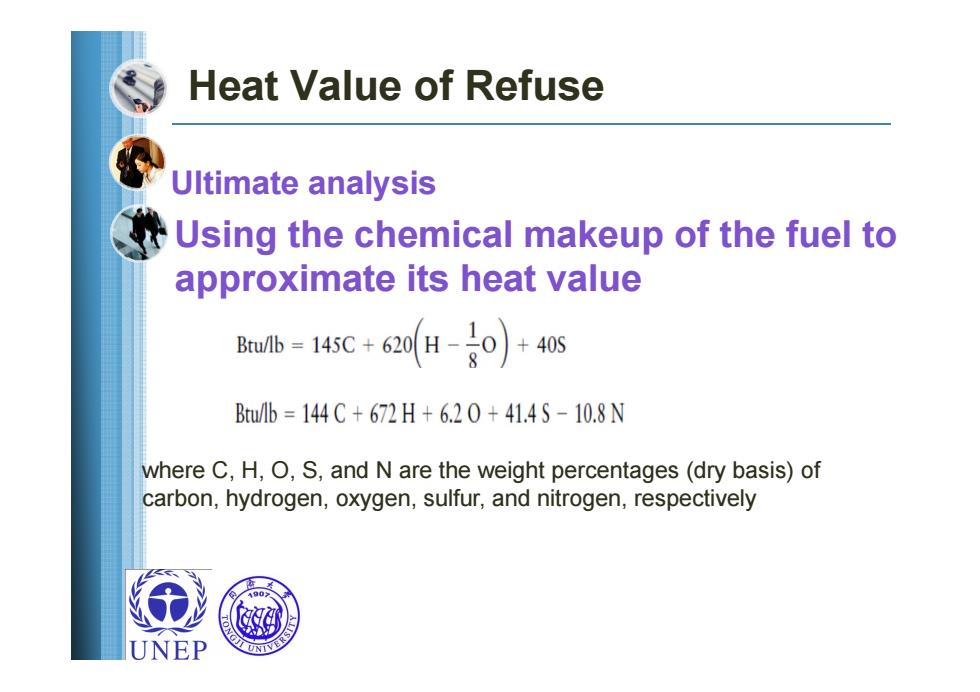
Heat Value of Refuse Ultimate analysis Using the chemical makeup of the fuel to approximate its heat value Bib=145c+620H-0)+40s Bu/1b=144C+672H+6.20+41.4S-10.8N where C,H,O,S,and N are the weight percentages(dry basis)of carbon,hydrogen,oxygen,sulfur,and nitrogen,respectively UNEP
Heat Value of Refuse Ultimate analysis Using the chemical makeup of the fuel to approximate its heat value where C, H, O, S, and N are the weight percentages (dry basis) of carbon, hydrogen, oxygen, sulfur, and nitrogen, respectively

Heat Value of Refuse Compositional analyses Bu/b=49R+22.5G+P)-3.3W Using regression analysis and comparing the results to actual measurements of heat value Bu/l=1238+15.6R+4.4P+2.7G-20.7W R-plastics,percent by weight,on dry basis P- paper,percent by weight,on dry basis G food wastes,percent by weight,on dry basis water,percent by weight,on dry basis UNEP
Heat Value of Refuse Compositional analyses Using regression analysis and comparing the results to actual measurements of heat value R= plastics, percent by weight, on dry basis P= paper, percent by weight, on dry basis G= food wastes, percent by weight, on dry basis W= water, percent by weight, on dry basis
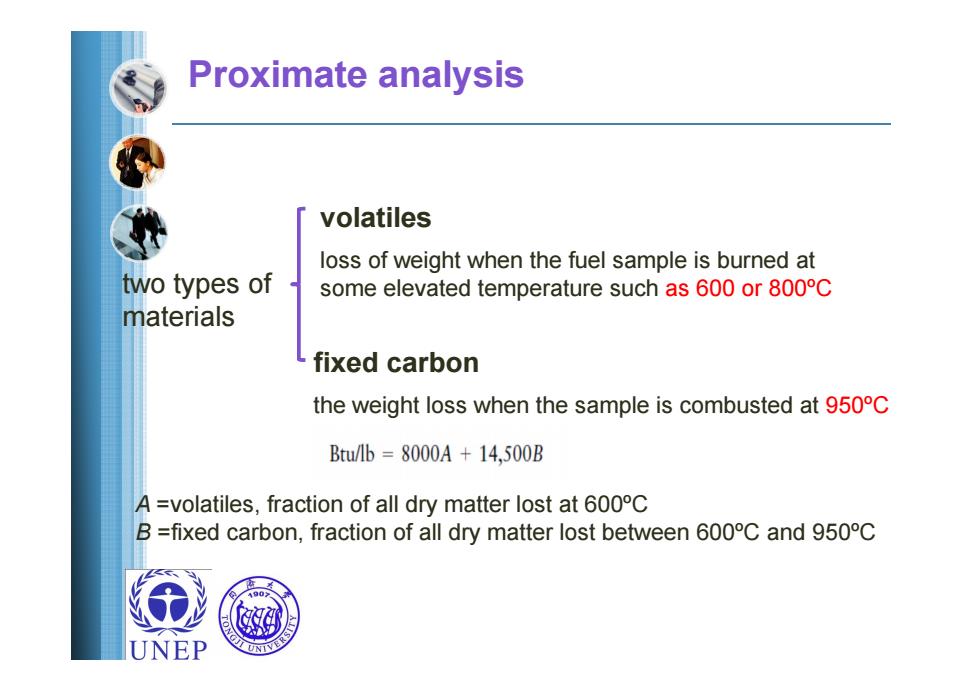
Proximate analysis volatiles loss of weight when the fuel sample is burned at two types of some elevated temperature such as 600 or 800C materials fixed carbon the weight loss when the sample is combusted at 950C B/l=8000A+14,500B A =volatiles,fraction of all dry matter lost at 600C B =fixed carbon,fraction of all dry matter lost between 600C and 950C UNEP
Proximate analysis volatiles fixed carbon loss of weight when the fuel sample is burned at some elevated temperature such as 600 or 800ºC the weight loss when the sample is combusted at 950ºC two types of materials A =volatiles, fraction of all dry matter lost at 600ºC B =fixed carbon, fraction of all dry matter lost between 600ºC and 950ºC
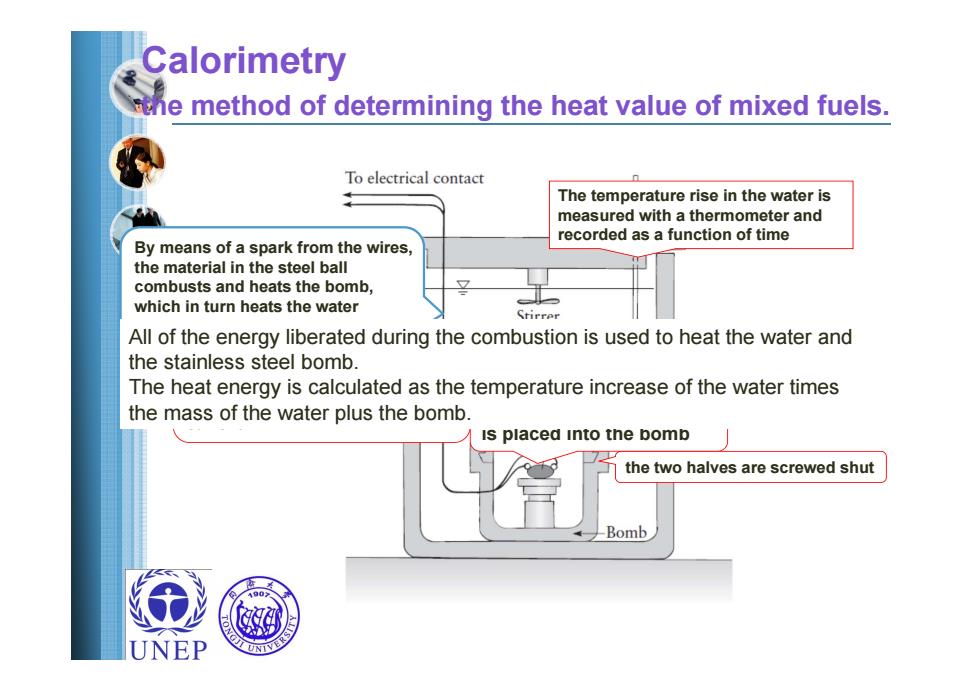
Calorimetry the method of determining the heat value of mixed fuels. To electrical contact The temperature rise in the water is measured with a thermometer and recorded as a function of time By means of a spark from the wires, the material in the steel ball combusts and heats the bomb, which in turn heats the water Stirrer All of the energy liberated during the combustion is used to heat the water and the stainless steel bomb The heat energy is calculated as the temperature increase of the water times the mass of the water plus the bomb is placed into the bomb the two halves are screwed shut -Bomb UNEP
Calorimetry the method of determining the heat value of mixed fuels. A sample of known weight is placed into the bomb the two halves are screwed shut By means of a spark from the wires, the material in the steel ball combusts and heats the bomb, which in turn heats the water Oxygen under high pressure the bomb is placed in an adiabatic is then injected into the bomb water bath with wires leading from the bomb to a source of electrical current The temperature rise in the water is measured with a thermometer and recorded as a function of time All of the energy liberated during the combustion is used to heat the water and the stainless steel bomb. The heat energy is calculated as the temperature increase of the water times the mass of the water plus the bomb
An important aspect of calorimetric heat values higher heating value (HHV) the net calorific energy The HHV is calculated by including the contribution due to the distinction this latent heat of vaporization(the heat required to produce steam from water) lower heating value(LHV) the gross calorific energy The LHV is then the HHV minus the latent heat of vaporization that has occurred in the bomb calorimeter UNEP
the gross calorific energy the net calorific energy the distinction higher heating value(HHV) lower heating value(LHV) The HHV is calculated by including the contribution due to this latent heat of vaporization (the heat required to produce steam from water) The LHV is then the HHV minus the latent heat of vaporization that has occurred in the bomb calorimeter An important aspect of calorimetric heat values
why the HHV number overestimates the actual heat value 1. the presence of metals temperature maybe not sufficiently high to force the reaction, like the oxidation of aluminum 2. the incomplete combustion of organics while all organic material will oxidize in a calorimeter,this will not occur in a full-scale combustor. The amount of unburned organics can vary from 2 to 25%, depending on the effectiveness of the operation Q龙 UNEP
1. the presence of metals 2. the incomplete combustion of organics temperature maybe not sufficiently high to force the reaction, like the oxidation of aluminum while all organic material will oxidize in a calorimeter, this will not occur in a full-scale combustor. The amount of unburned organics can vary from 2 to 25%, depending on the effectiveness of the operation why the HHV number overestimates the actual heat value