正在加载图片...
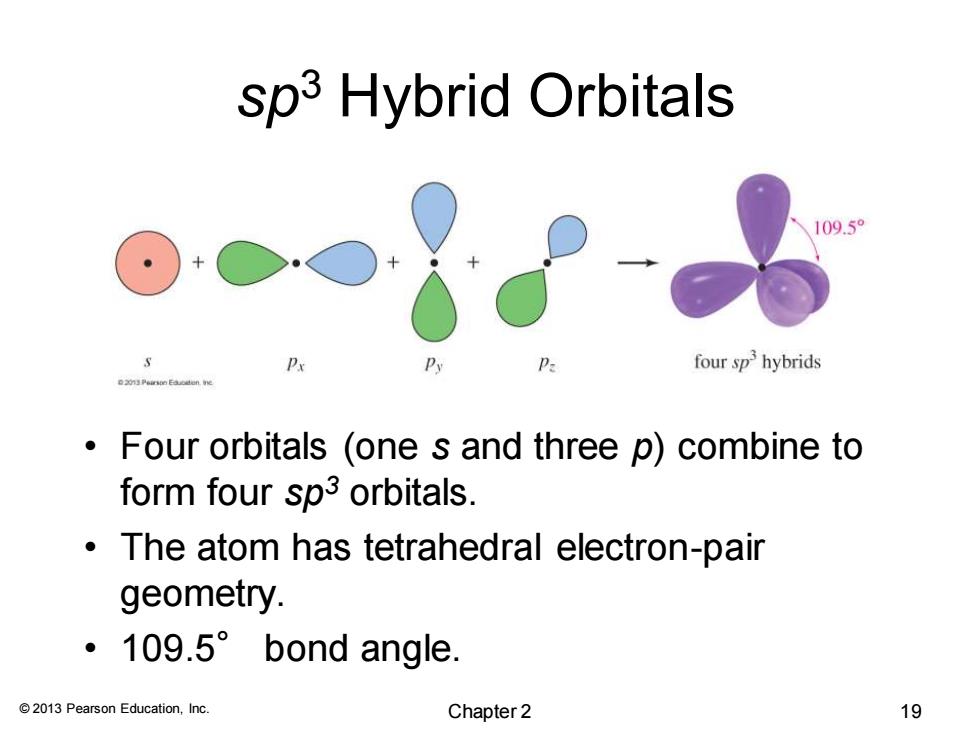
sp3 Hybrid Orbitals 109.5° P four sp hybrids Peursn Eaodon ne Four orbitals (one s and three p)combine to form four sp3 orbitals. The atom has tetrahedral electron-pair geometry. ·109.5°bond angle. 2013 Pearson Education,Inc. Chapter 2 19© 2013 Pearson Education, Inc. sp3 Hybrid Orbitals • Four orbitals (one s and three p) combine to form four sp3 orbitals. • The atom has tetrahedral electron-pair geometry. • 109.5° bond angle. Chapter 2 19