正在加载图片...
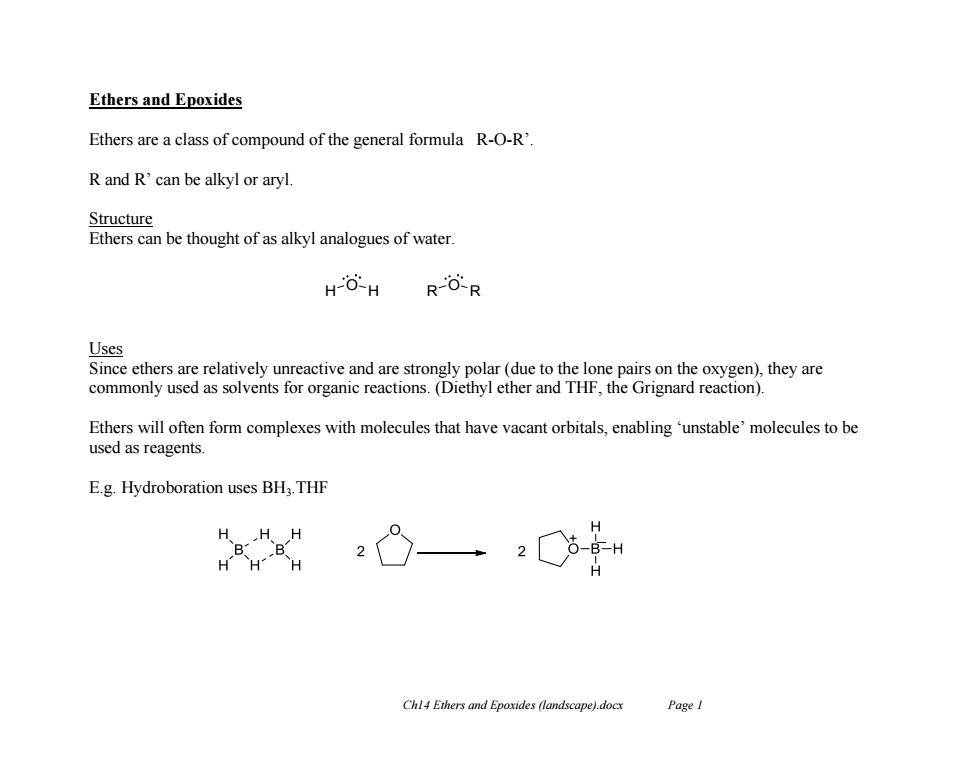
Ethers and Epoxides Ethers are a class of compound of the general formula R-O-R'. R and R'can be alkyl or aryl Structure Ethers can be thought of as alkyl analogues of water HO-H RO-R Uses Since ethers are relatively unreactive and are strongly polar(due to the lone pairs on the oxygen),they are commonly used as solvents for organic reactions.(Diethyl ether and THF,the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals,enabling'unstable'molecules to be used as reagents. E.g.Hydroboration uses BH:.THF ”g HHH Chl4 Ethers and Epoxides (landscape).docx Page ICh14 Ethers and Epoxides (landscape).docx Page 1 Ethers and Epoxides Ethers are a class of compound of the general formula R-O-R’. R and R’ can be alkyl or aryl. Structure Ethers can be thought of as alkyl analogues of water. Uses Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable’ molecules to be used as reagents. E.g. Hydroboration uses BH3.THF H O H R O R