正在加载图片...
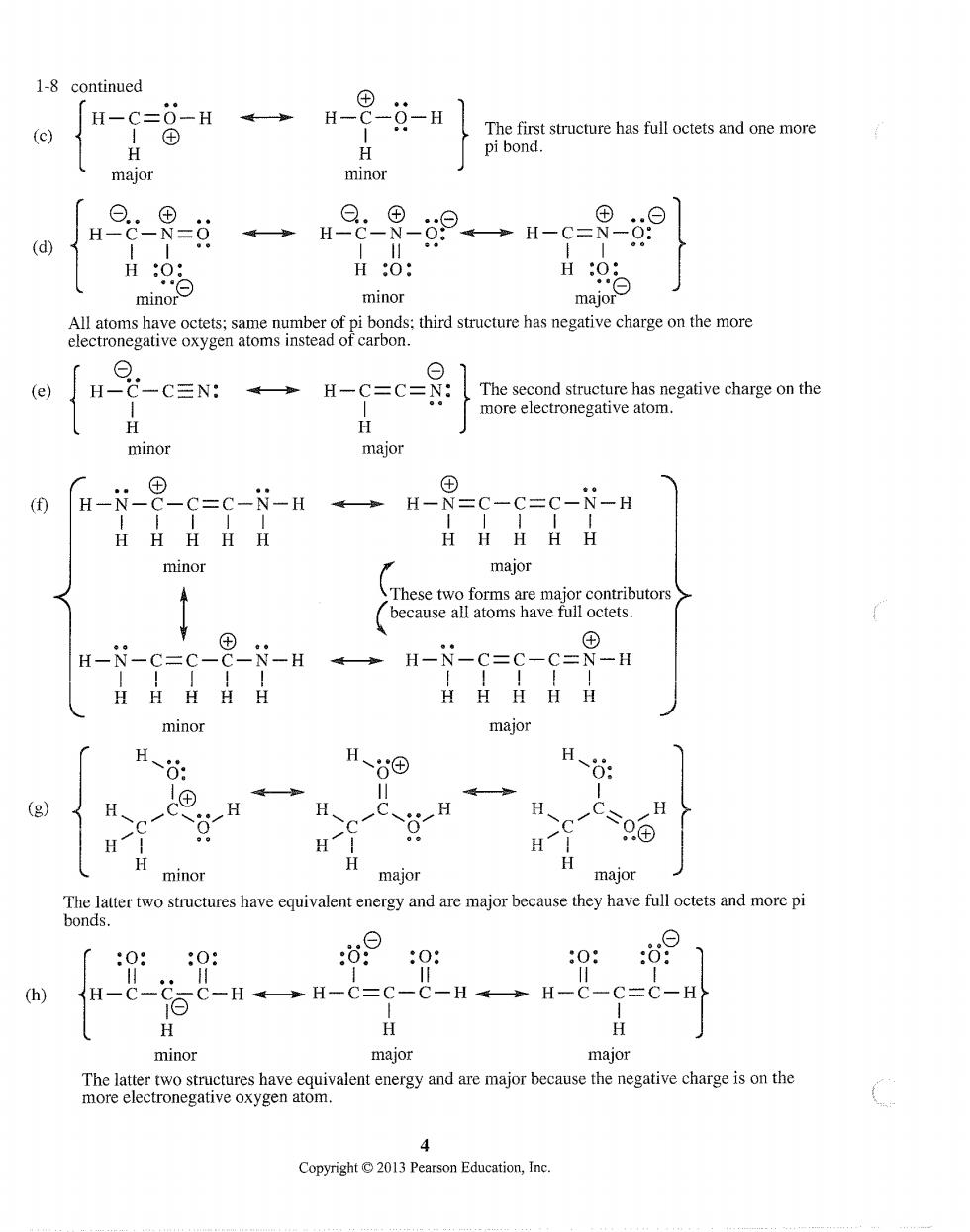
1-8 continued 「H-c=-H→H-8-g-H (c) The first structure has full octets and one more pi bond. minor 9→H-c=9- (d) minor major havecmbonds:thsr has negative charge electronegative oxygen atoms instea H-C-CEN: H-C=C=N: The second structure has negative charge on the more electronegative atom. minor major H-N_ -c=C-N-H→ H HHHHH minor major H-N-C=c- -H4→ HHHHH minor major H、 H、⊙ 日-6 H H H、 H H H H minor major major The latter two structures have equivalent energy and are major because they have full octets and more pi bonds. 0 99 (h) H-C-C_ -H→H-C=c-C-H→H-C-C=C-H H H H minor major major The latter two structures have equivalent energy and are major because the negative charge is on the more electronegative oxygen atom. Copyright013 Pearson Education,Ine