正在加载图片...
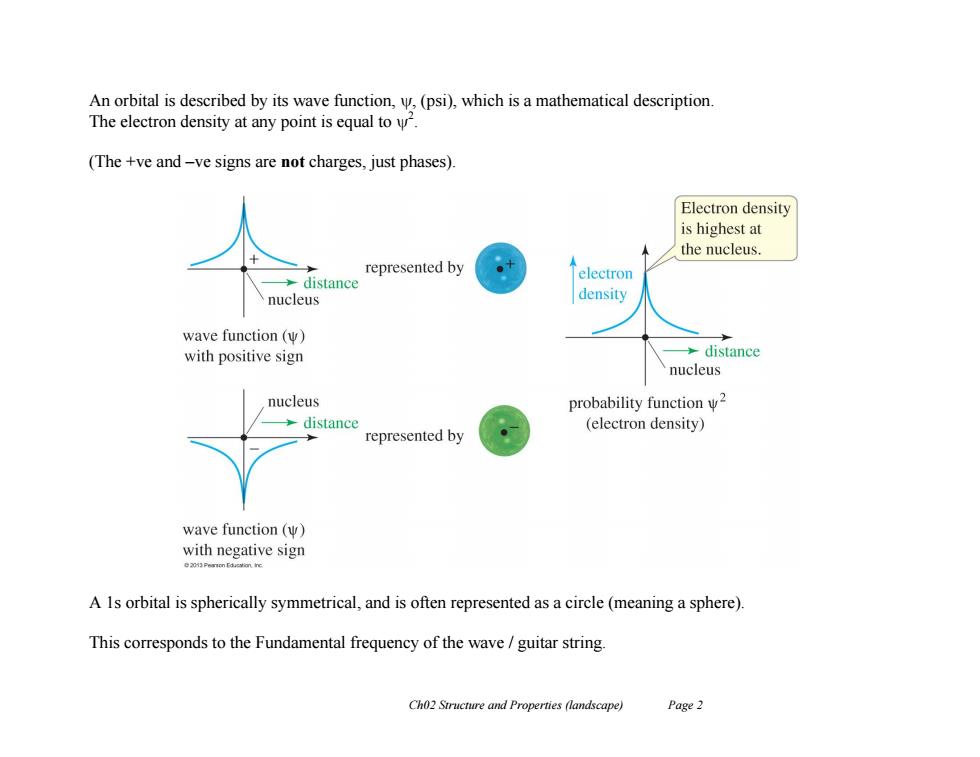
An orbital is described by its wave function,,(psi),which is a mathematical description The electron density at any point is equal to. (The +ve and-ve signs are not charges,just phases). Electron density is highest at the nucleus represented by distance electron nucleus density wave function(w) with positive sign >distance nucleus nucleus probability function w2 distance (electron density) represented by wave function (W) with negative sign A 1s orbital is spherically symmetrical,and is often represented as a circle(meaning a sphere). This corresponds to the Fundamental frequency of the wave guitar string Ch02 Structure and Properties (landscape) Page 2 Ch02 Structure and Properties (landscape) Page 2 An orbital is described by its wave function, , (psi), which is a mathematical description. The electron density at any point is equal to 2 . (The +ve and –ve signs are not charges, just phases). A 1s orbital is spherically symmetrical, and is often represented as a circle (meaning a sphere). This corresponds to the Fundamental frequency of the wave / guitar string