正在加载图片...
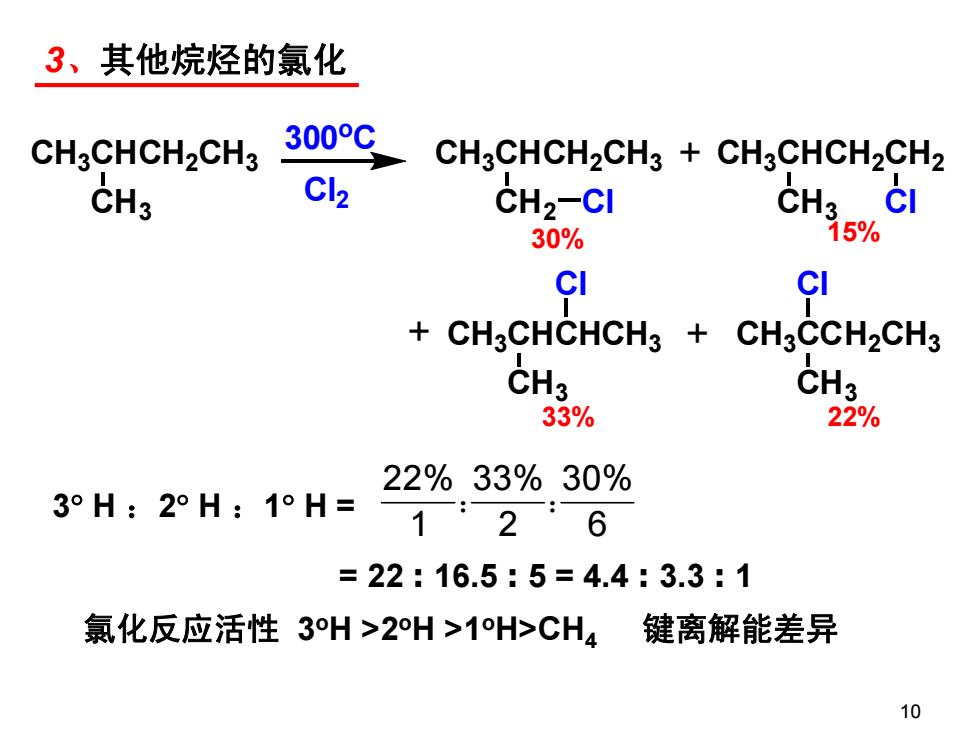
3、其他烷烃的氯化 CH3CHCH2CH3 300℃ CH3CHCH2CH3 CH3CHCH2CH2 CH3 Cl2 CH2-CI CH3 30% 5% CI CI + CH3CHCHCH3 CH3CCH2CH3 CH3 CH3 33% 22% 22%33%30% 3°H:2°H:1°H= 126 =22:16.5:5=4.4:3.3:1 氯化反应活性3H>2H>1H>CH4 键离解能差异 103、其他烷烃的氯化 3 H :2 H :1 H = 22% 33% 30% 126 : : = 22 : 16.5 : 5 = 4.4 : 3.3 : 1 氯化反应活性 3oH >2oH >1oH>CH4 键离解能差异 10 CH3CHCH2CH3 Cl2 300oC + CH3 CH3CHCH2CH3 CH2 CH3CHCH2CH2 CH3 CH3CHCHCH3 CH3 CH3CCH2CH3 CH3 Cl Cl Cl Cl + + 30% 15% 33% 22%