正在加载图片...
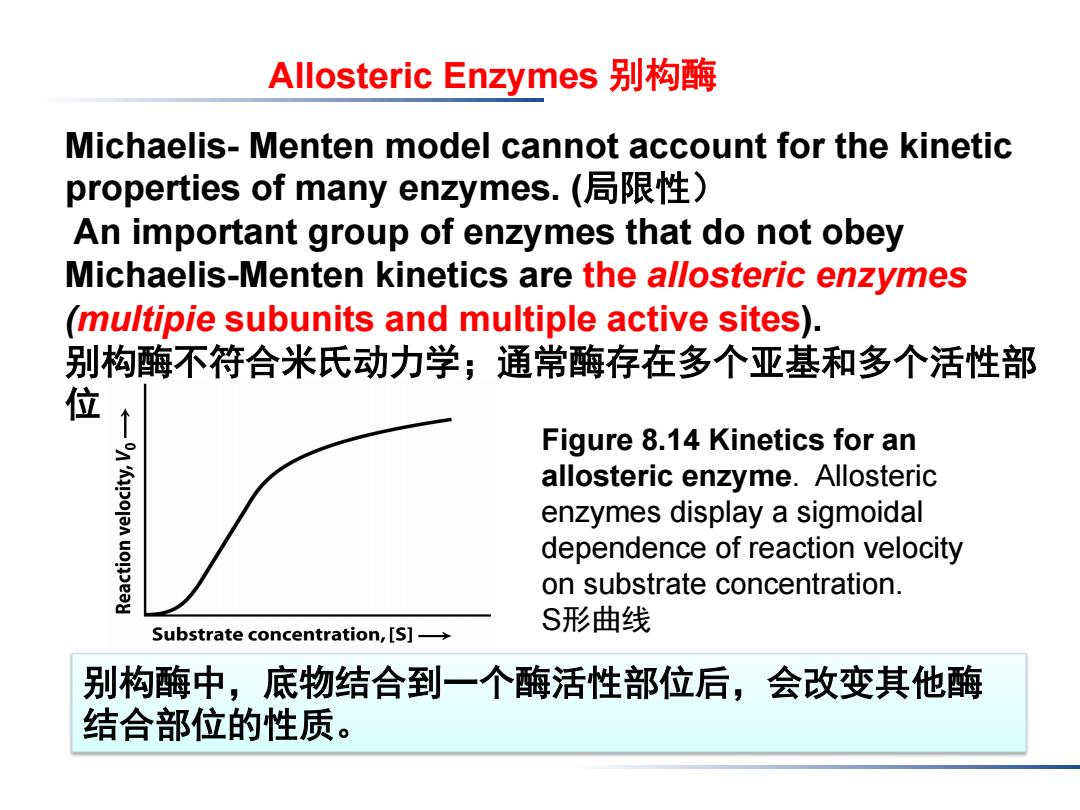
Allosteric Enzymes别构酶 Michaelis-Menten model cannot account for the kinetic properties of many enzymes.(局限性) An important group of enzymes that do not obey Michaelis-Menten kinetics are the allosteric enzymes (mu/tipie subunits and multiple active sites). 别构酶不符合米氏动力学;通常酶存在多个亚基和多个活性部 位 Figure 8.14 Kinetics for an allosteric enzyme.Allosteric enzymes display a sigmoidal dependence of reaction velocity on substrate concentration. S形曲线 Substrate concentration,[S]- 别构酶中,底物结合到一个酶活性部位后,会改变其他酶 结合部位的性质。Figure 8.14 Kinetics for an allosteric enzyme. Allosteric enzymes display a sigmoidal dependence of reaction velocity on substrate concentration. S形曲线 Allosteric Enzymes 别构酶 Michaelis- Menten model cannot account for the kinetic properties of many enzymes. (局限性) An important group of enzymes that do not obey Michaelis-Menten kinetics are the allosteric enzymes (multipie subunits and multiple active sites). 别构酶不符合米氏动力学;通常酶存在多个亚基和多个活性部 位 别构酶中,底物结合到一个酶活性部位后,会改变其他酶 结合部位的性质