正在加载图片...
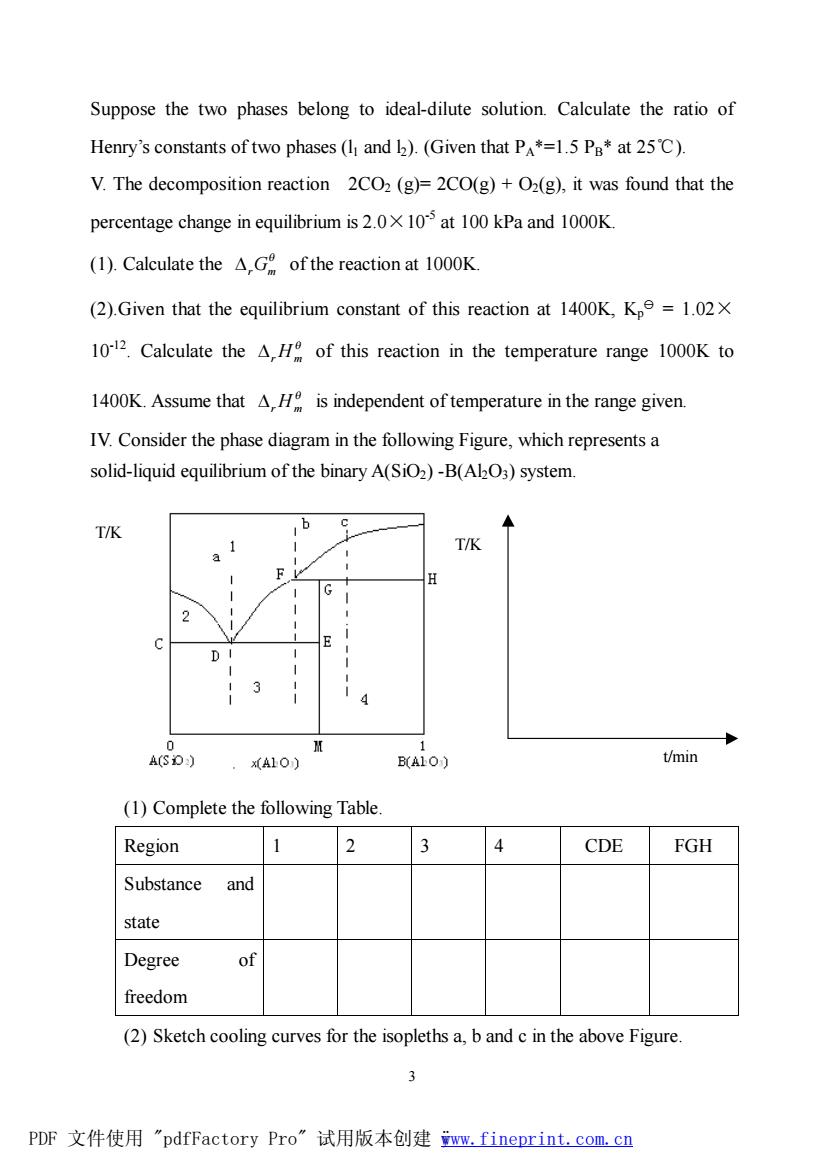
Suppose the two phases belong to ideal-dilute solution.Calculate the ratio of Henry's constants of two phases (I and )(Given that P*=1.5 Pa*at 25C). V.The decomposition reaction 2CO2(g)=2CO(g)+O2(g),it was found that the percentage change in equilibrium is 2.010 at 100 kPa and 1000K. (1).Calculate the AG of the reaction at 1000K (2).Given that the equilibrium constant of this reaction at 1400K,Kp=1.02X 1012.Calculate the H of this reaction in the temperature range 1000K to 1400K.Assume that AH is independent of temperature in the range given IV.Consider the phase diagram in the following Figure,which represents a solid-liquid equilibrium of the binary A(SiO2)-B(AkO3)system T/K T/K D A10) B(ALO) t/min (1)Complete the following Table Region 2 4 CDE FGH Substance and state Degree of freedom (2)Sketch cooling curves for the isopleths a,b and c in the above Figure. 3 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn 3 Suppose the two phases belong to ideal-dilute solution. Calculate the ratio of Henry’s constants of two phases (l1 and l2). (Given that PA*=1.5 PB* at 25℃). V. The decomposition reaction 2CO2 (g)= 2CO(g) + O2(g), it was found that the percentage change in equilibrium is 2.0×10-5 at 100 kPa and 1000K. (1). Calculate the q DrGm of the reaction at 1000K. (2).Given that the equilibrium constant of this reaction at 1400K, Kp y = 1.02× 10-12 . Calculate the q DrHm of this reaction in the temperature range 1000K to 1400K. Assume that q DrHm is independent of temperature in the range given. IV. Consider the phase diagram in the following Figure, which represents a solid-liquid equilibrium of the binary A(SiO2) -B(Al2O3) system. (1) Complete the following Table. Region 1 2 3 4 CDE FGH Substance and state Degree of freedom (2) Sketch cooling curves for the isopleths a, b and c in the above Figure. t/min T/K T/K PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn