正在加载图片...
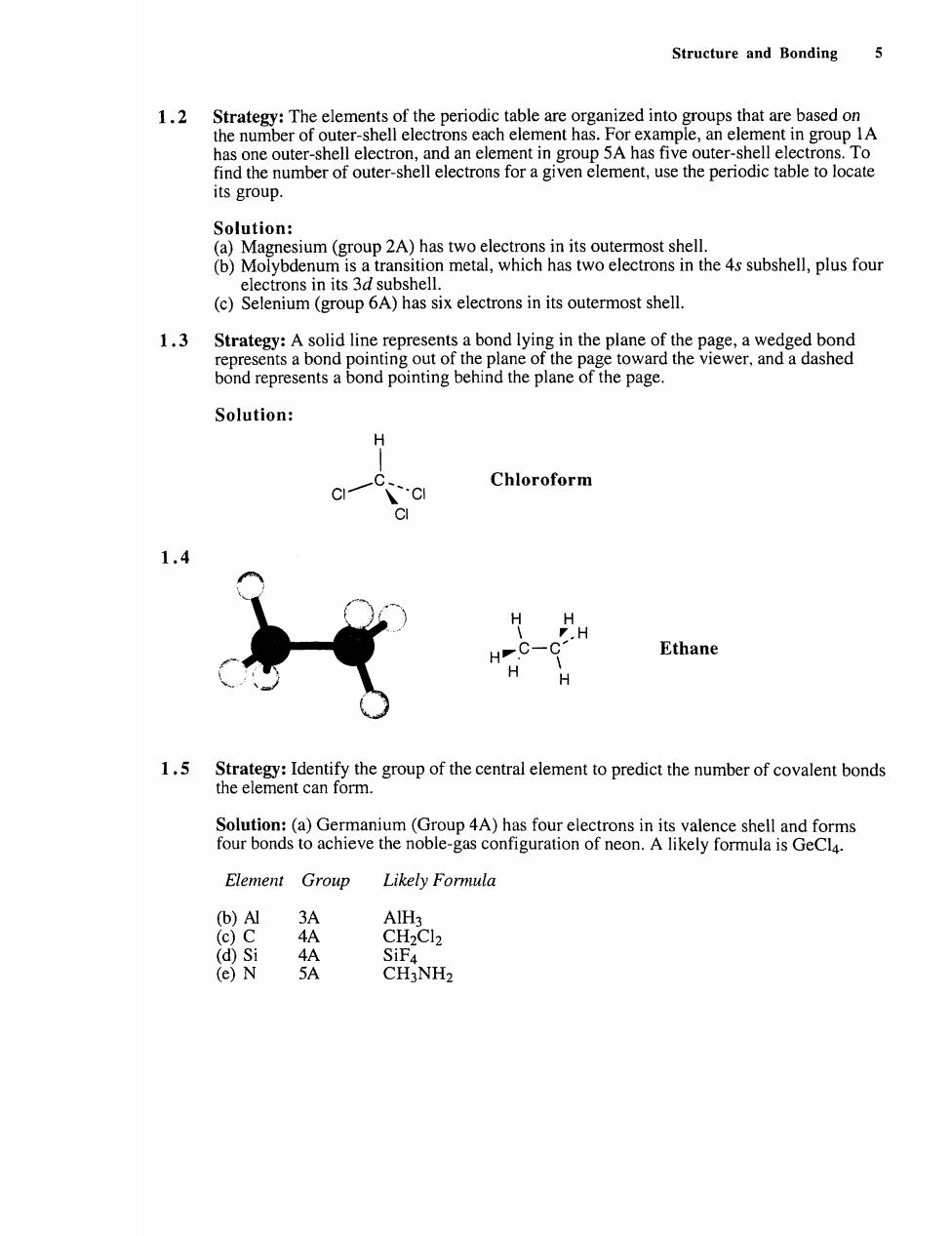
Structure and Bonding 5 1.2 he me o n h ent in group IA Solution: ons in its 3d subshell. (c)Selenium(group 6A)has six electrons in its outermost shell. 1.3 Strategy:bond A solid li ts a bond lyi bndbond pontingnd the pofpage. Solution: H CI- Chloroform 1.d H PH Ethane H 1.5 Identify the group of the central element to predict the number of covalent bonds Element Group Likely Formula (b)Al (d)Si SiF (e)N 5A CH3NH2