正在加载图片...
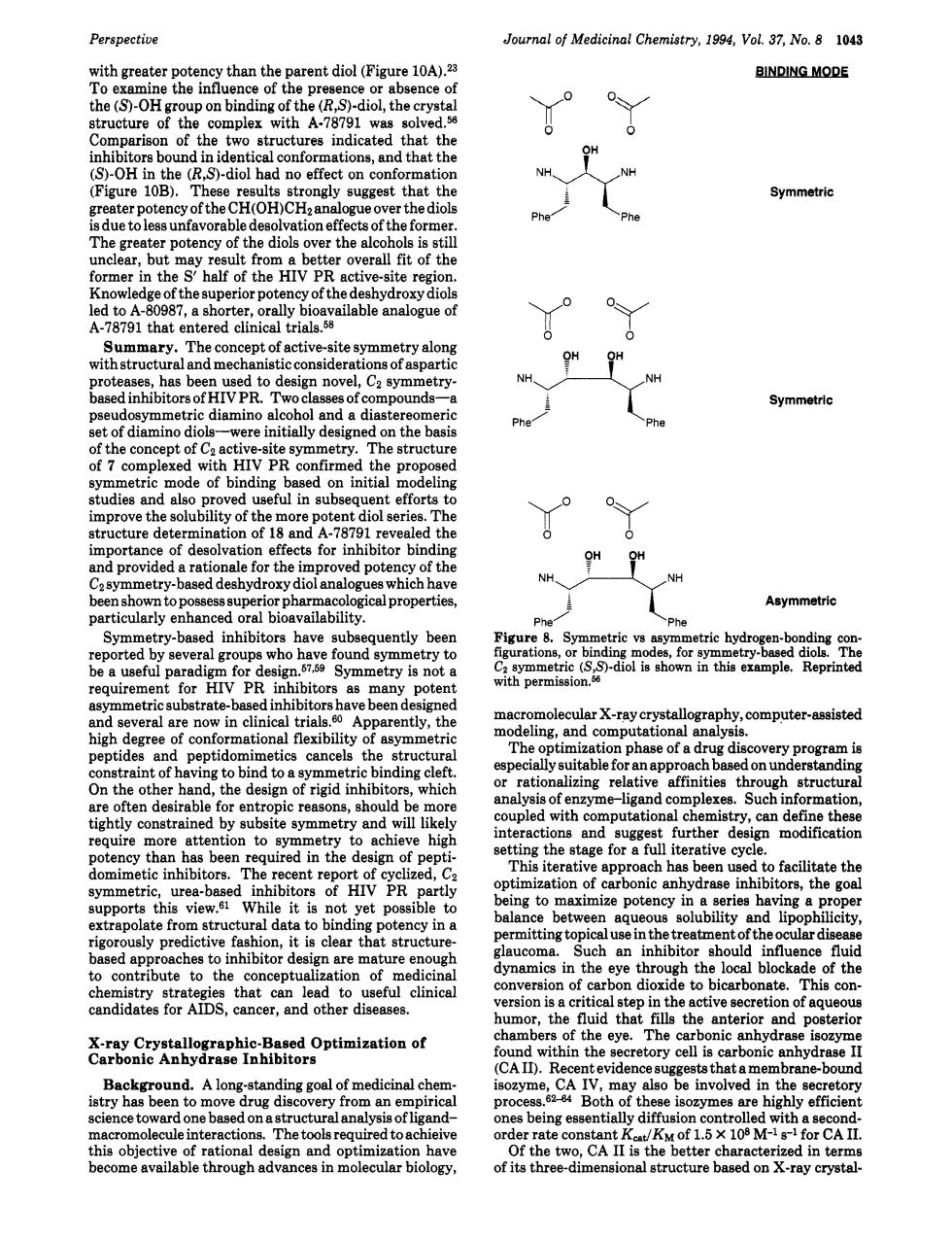
Perspective Journal of Medicinal Chemistry,1994,VoL 37,No.8 1043 BINDING MODE the cry ndicated that the nations,and that the NH Figure 10B). 602otencyorthey gest that the Symmetric (OH)CH,n siteregioh nowl 0 entered clinica tri The concept of active-ite ay metry along tru has be etry NH Symmetric of diamino di ereinitialydesi on the basi Phe Caitriandeofbdngbaedhit ore oten diol serie atructurecdeg inhibitor bin provided arat o for the Asymmetric Symmetry is not dinhibitorsh e been onal flexibility The zation phase of a drug di cancels the very programs d,the dei hib which h in rmation metry and will inte and su equire ention,to ymmetry achieve hig setting stage for a “Th recent rep ed.C opt ization of carbonic anhydrasein bitors.the goa ate fr struc ncy in ugh seinthereantoud the e m ure contribute dynamics in the eye through the local blockade of the to the candidates for AIDS,cancer,and other disease on语ag ticals humor,the d that fills the anterio and terio &arBaSaaraieiOptinmzatioaot hin the retory cell is rbon canhydrase Background.A long-standing goal of medicinal che CA IV. ma lsobe involved in the to move pro effici s heing 41 lecule he tools re red toac rate cons the01.5×108M A de of its three-dimensional structure based on x-ray crystal Perspective with greater potency than the parent diol (Figure 10ALZ3 To examine the influence of the presence or absence of the (5')-OH group on binding of the (R,S)-diol, the crystal structure of the complex with A-78791 was solved.56 Comparison of the two structures indicated that the inhibitors bound in identical conformations, and that the (S)-OH in the (R,S)-diol had no effect on conformation (Figure 10B). These results strongly suggest that the greater potency of the CH(OH)CH2 analogue over the diols is due to less unfavorable desolvation effects of the former. The greater potency of the diols over the alcohols is still unclear, but may result from a better overall fit of the former in the S' half of the HIV PR active-site region. Knowledge of the superior potency of the deshydroxy diols led to A-80987, a shorter, orally bioavailable analogue of A-78791 that entered clinical trials.58 Summary. The concept of active-site symmetry along with structural and mechanistic considerations of aspartic proteases, has been used to design novel, CZ symmetrybased inhibitors of HIV PR. Two classes of compounds-a pseudosymmetric diamino alcohol and a diastereomeric set of diamino diols-were initially designed on the basis of the concept of CZ active-site symmetry. The structure of 7 complexed with HIV PR confirmed the proposed symmetric mode of binding based on initial modeling studies and also proved useful in subsequent efforts to improve the solubility of the more potent diol series. The structure determination of 18 and A-78791 revealed the importance of desolvation effects for inhibitor binding and provided a rationale for the improved potency of the CZ symmetry-based deshydroxy diol analogues which have been shown to possess superior pharmacological properties, particularly enhanced oral bioavailability. Symmetry-based inhibitors have subsequently been reported by several groups who have found symmetry to be a useful paradigm for de~ign.~'~~~ Symmetry is not a requirement for HIV PR inhibitors as many potent asymmetric substrate-based inhibitors have been designed and several are now in clinical trials.60 Apparently, the high degree of conformational flexibility of asymmetric peptides and peptidomimetics cancels the structural constraint of having to bind to a symmetric binding cleft. On the other hand, the design of rigid inhibitors, which are often desirable for entropic reasons, should be more tightly constrained by subsite symmetry and will likely require more attention to symmetry to achieve high potency than has been required in the design of peptidomimetic inhibitors. The recent report of cyclized, CZ symmetric, urea-based inhibitors of HIV PR partly supports this view.61 While it is not yet possible to extrapolate from structural data to binding potency in a rigorously predictive fashion, it is clear that structurebased approaches to inhibitor design are mature enough to contribute to the conceptualization of medicinal chemistry strategies that can lead to useful clinical candidates for AIDS, cancer, and other diseases. Journal of Medicinal Chemistry, 1994, Vol. 37, No. 8 1043 X-ray Crystallographic-Based Optimization of Carbonic Anhydrase Inhibitors Background. A long-standing goal of medicinal chemistry has been to move drug discovery from an empirical science toward one based on a structural analysis of ligandmacromolecule interactions. The tools required to achieive this objective of rational design and optimization have become available through advances in molecular biology, Yo 0 OY 0 Symmetric Phej 'Phe Symmetric Yo 0 OY 0 NHyo" Of NH PheJ Phe Asymmetric Figure 8. Symmetric vs asymmetric hydrogen-bonding configurations, or binding modes, for symmetry-based diols. The Cp symmetric (S,S)-diol is shown in this example. Reprinted with permissi0n.W macromolecular X-ray crystallography, computer-assisted modeling, and computational analysis. The optimization phase of a drug discovery program is especially suitable for an approach based on understanding or rationalizing relative affinities through structural analysis of enzyme-ligand complexes. Such information, coupled with computational chemistry, can define these interactions and suggest further design modification setting the stage for a full iterative cycle. This iterative approach has been used to facilitate the optimization of carbonic anhydrase inhibitors, the goal being to maximize potency in a series having a proper balance between aqueous solubility and lipophilicity, permitting topical use in the treatment of the ocular disease glaucoma. Such an inhibitor should influence fluid dynamics in the eye through the local blockade of the conversion of carbon dioxide to bicarbonate. This conversion is a critical step in the active secretion of aqueous humor, the fluid that fills the anterior and posterior chambers of the eye. The carbonic anhydrase isozyme found within the secretory cell is carbonic anhydrase I1 (CA 11). Recent evidence suggests that amembrane-bound isozyme, CA IV, may also be involved in the secretory process.62-64 Both of these isozymes are highly efficient ones being essentially diffusion controlled with a secondorder rate constant K,JKM of 1.5 X lo8 M-l s-1 for CA 11. Of the two, CA I1 is the better characterized in terms of its three-dimensional structure based on X-ray crystal-