正在加载图片...
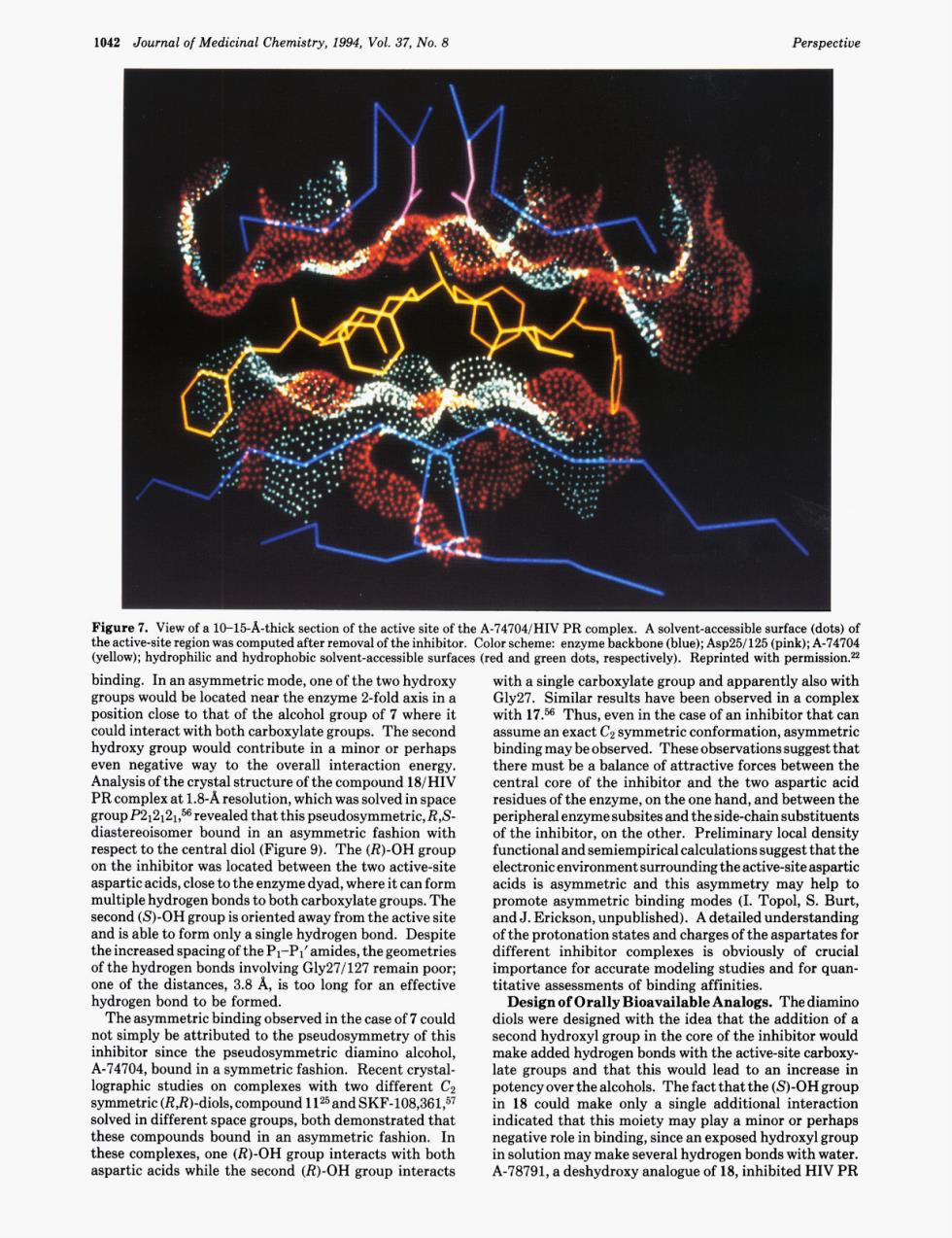
1042 Journal of Medicinal Chemistry,1994,Vol.37,No.8 hy red and green dots ermission en in the The seconc ll interactior d18/H there must be he ve forcesbe en the en the revealed his pseud pesidechainsubstituents gure 9).The (R)-OH group functional and semiempirical calcula ionss st that the rticacids,Cow tTrhndng etry may nds The promote binding modes en b nd.Des spartates fo trie ortance for ac tit edng ob ric fas Rec late groups n18 alcohoihia only single may play a m H 心tion may makese一”名8安 roxy a1042 Journal of Medicinal Chemistry, 1994, Vol. 37, No. 8 Perspective Figure 7. View of a 10-15-A-thick section of the active site of the A-74704/HIV PR complex. A solvent-accessible surface (dots) of the active-site region was computed after removal of the inhibitor. Color scheme: enzyme backbone (blue); Asp25/ 125 (pink); A-74704 (yellow); hydrophilic and hydrophobic solvent-accessible surfaces (red and green dots, respectively). Reprinted with permission.22 binding. In an asymmetric mode, one of the two hydroxy groups would be located near the enzyme 2-fold axis in a position close to that of the alcohol group of 7 where it could interact with both carboxylate groups. The second hydroxy group would contribute in a minor or perhaps even negative way to the overall interaction energy. Analysis of the crystal structure of the compound 18/HIV PR complex at 1.8-A resolution, which was solved in space gro~pP212121,~~ revealed that this pseudosymmetric, R,Sdiastereoisomer bound in an asymmetric fashion with respect to the central diol (Figure 9). The @)-OH group on the inhibitor was located between the two active-site aspartic acids, close to the enzyme dyad, where it can form multiple hydrogen bonds to both carboxylate groups. The second (S)-OH group is oriented away from the active site and is able to form only a single hydrogen bond. Despite the increased spacing of the Pl-Pl’ amides, the geometries of the hydrogen bonds involving Gly27/127 remain poor; one of the distances, 3.8 A, is too long for an effective hydrogen bond to be formed. The asymmetric binding observed in the case of 7 could not simply be attributed to the pseudosymmetry of this inhibitor since the pseudosymmetric diamino alcohol, A-74704, bound in a symmetric fashion. Recent crystallographic studies on complexes with two different C2 symmetric (R,R)-diols, compound 1 125 and SKF-108,361,57 solved in different space groups, both demonstrated that these compounds bound in an asymmetric fashion. In these complexes, one (&)-OH group interacts with both aspartic acids while the second (R)-OH group interacts with a single carboxylate group and apparently also with Gly27. Similar results have been observed in a complex with 17.56 Thus, even in the case of an inhibitor that can assume an exact C2 symmetric conformation, asymmetric binding may be observed. These observations suggest that there must be a balance of attractive forces between the central core of the inhibitor and the two aspartic acid residues of the enzyme, on the one hand, and between the peripheral enzyme subsites and the side-chain substituents of the inhibitor, on the other. Preliminary local density functional and semiempirical calculations suggest that the electronic environment surrounding the active-site aspartic acids is asymmetric and this asymmetry may help to promote asymmetric binding modes (I. Topol, S. Burt, and J. Erickson, unpublished). A detailed understanding of the protonation states and charges of the aspartates for different inhibitor complexes is obviously of crucial importance for accurate modeling studies and for quantitative assessments of binding affinities. Design of Orally Bioavailable Analogs. The diamino diols were designed with the idea that the addition of a second hydroxyl group in the core of the inhibitor would make added hydrogen bonds with the active-site carboxylate groups and that this would lead to an increase in potency over the alcohols. The fact that the @)-OH group in 18 could make only a single additional interaction indicated that this moiety may play a minor or perhaps negative role in binding, since an exposed hydroxyl group in solution may make several hydrogen bonds with water. A-78791, a deshydroxy analogue of 18, inhibited HIV PR