正在加载图片...
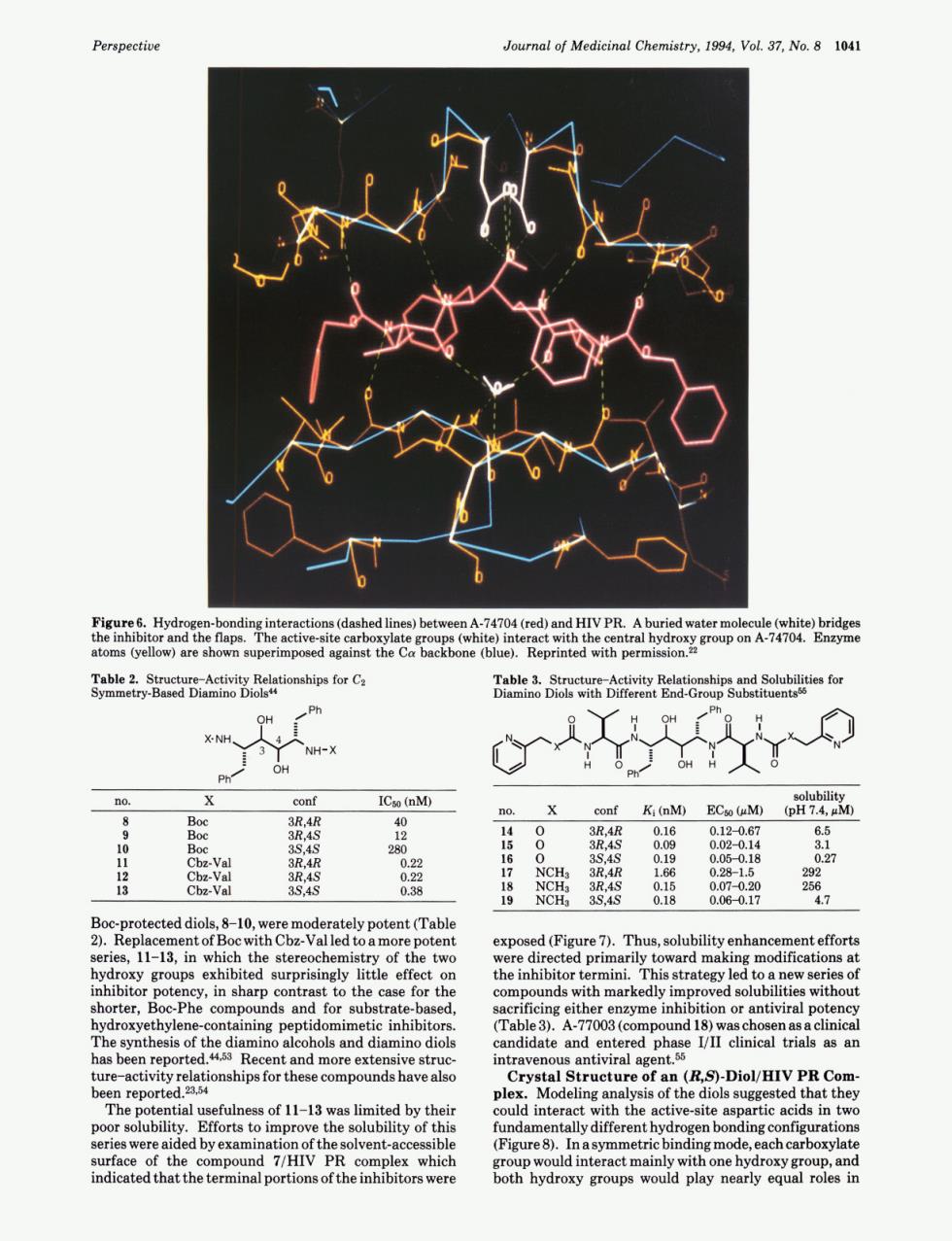
Perspective Journal of Medicinal Chemistry,1994,Vol.37,No.8 1041 gahe子sgastaiogtoamhpetacc a品 conf ICso (nM) no 3 conr Ki (mM)ECa (eM)( 2 0.2 he two expos sed (Figure7).Thus,solubility enhancement effort d primarily toward mak candidate and Crystal Structure of a (R.S)-Diol/HIV PR Com plex. tyof this ren bonding c surface of the d7/HIV PR complex which oun and indicated that the terminal portions of the inhibitors were Perspective Journal of Medicinal Chemistry, 1994, Vol. 37, No. 8 1041 I b Figure 6. Hydrogen-bonding interactions (dashed lines) between A-74704 (red) and HIV PR. A buried water molecule (white) bridges the inhibitor and the flaps. The active-site carboxylate groups (white) interact with the central hydroxy group on A-74704. Enzyme atoms (yellow) are shown superimposed against the Ca backbone (blue). Reprinted with permission.22 Table 2. Structure-Activity Relationships for Cz Symmetry-Based Diamino Diols4 OH OPh X- NH NH - OH Ph; Table 3. Structure-Activity Relationships and Solubilities for Diamino Diols with Different End-Group Substituents66 "K 0 solubility no. X conf Ki (nM) ECw(bM) (pH7.4,pM) ~ ~~~ ~ 8 Boc 3R,4R 40 10 Boc 3244s 280 9 Boc 3R,4S 12 11 Cbz-Val 3R,4R 0.22 12 Cbz-Val 3R,4S 0.22 13 C bz-Val 3s,4s 0.38 14 0 3R,4R 0.16 0.12-0.67 6.5 15 0 3R,4S 0.09 0.02-0.14 3.1 16 0 3S,4S 0.19 0.05-0.18 0.27 17 NCH3 3R,4R 1.66 0.28-1.5 292 18 NCH3 3R,4S 0.15 0.07-0.20 256 19 NCH2 3S,4S 0.18 0.06-0.17 4.7 Boc-protected diols, 8-10, were moderately potent (Table 2). Replacement of Boc with Cbz-Val led to a more potent series, 11-13, in which the stereochemistry of the two hydroxy groups exhibited surprisingly little effect on inhibitor potency, in sharp contrast to the case for the shorter, Boc-Phe compounds and for substrate-based, hydroxyethylene-containing peptidomimetic inhibitors. The synthesis of the diamino alcohols and diamino diols has been rep0rted.~*~3 Recent and more extensive structure-activity relationships for these compounds have also been rep~rted.~~,~~ The potential usefulness of 11-13 was limited by their poor solubility. Efforts to improve the solubility of this series were aided by examination of the solvent-accessible surface of the compound 7/HIV PR complex which indicated that the terminal portions of the inhibitors were exposed (Figure 7). Thus, solubility enhancement efforts were directed primarily toward making modifications at the inhibitor termini. This strategy led to a new series of compounds with markedly improved solubilities without sacrificing either enzyme inhibition or antiviral potency (Table 3). A-77003 (compound 18) was chosen as a clinical candidate and entered phase 1/11 clinical trials as an intravenous antiviral agent.55 Crystal Structure of an (B,S)-Diol/HIV PR Complex. Modeling analysis of the diols suggested that they could interact with the active-site aspartic acids in two fundamentally different hydrogen bonding configurations (Figure 8). In a symmetric binding mode, each carboxylate group would interact mainly with one hydroxy group, and both hydroxy groups would play nearly equal roles in