正在加载图片...
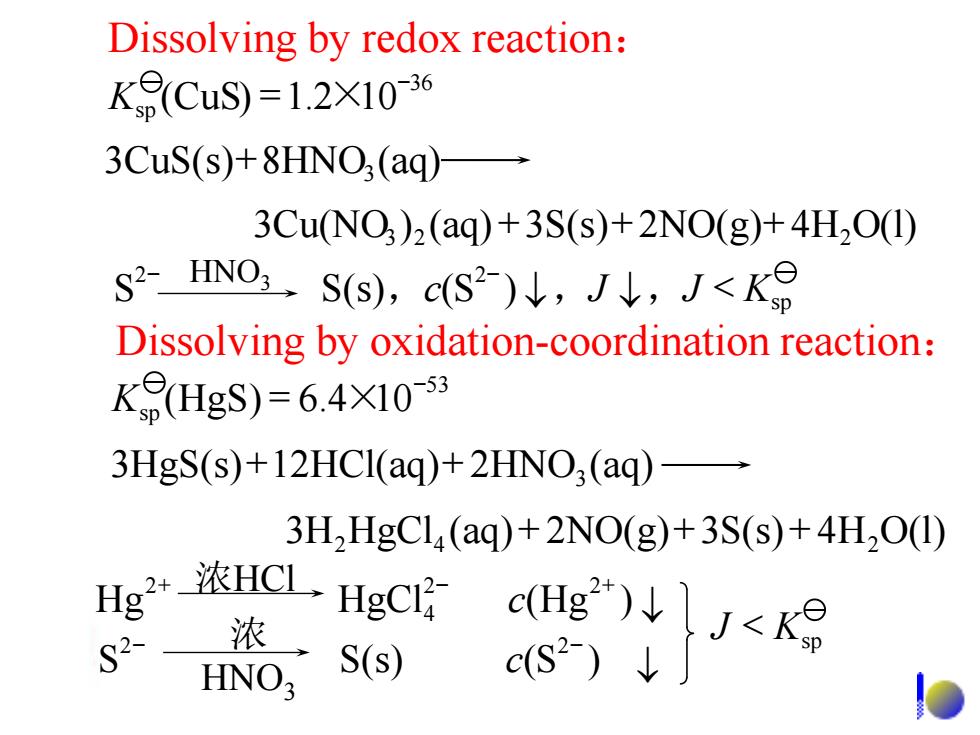
Dissolving by redox reaction: K9(CuS)=1.2X10-36 3CuS(s)+8HNO;(aq) 3Cu(NO )2(aq)+3S(s)+2NO(g)+4H2O(1) S2-HN0Ss),cS2)↓,J↓,J<K8 Dissolving by oxidation-coordination reaction: K8(HgS)=6.4X1053 3HgS(s)+12HCl(aq)+2HNO;(aq)- 3H2HgCl(aq)+2NO(g)+3S(s)+4H2O(1) CL) S2- 浓 HNO3 S(s) c(S2-)↓ Dissolving by oxidation-coordination reaction: 3 2 2 3Cu(NO ) (aq) +3S(s)+ 2NO(g)+ 4H O(l) 3H2HgCl4 (aq)+ 2NO(g)+ 3S(s)+ 4H2O(l) Dissolving by redox reaction: 36 Ksp (CuS) =1.2×10- 3 3CuS(s)+8HNO (aq) , , , sp 2 2 S S(s) c(S ) J J < K - HNO - 3 53 Ksp (HgS) = 6.4×10- 3 3HgS(s)+12HCl(aq)+ 2HNO (aq) 2 2 sp 2 2 4 2 S S(s) (S ) Hg HgCl (Hg ) J K c c < - - + 浓HCl - + 浓 HNO3