正在加载图片...
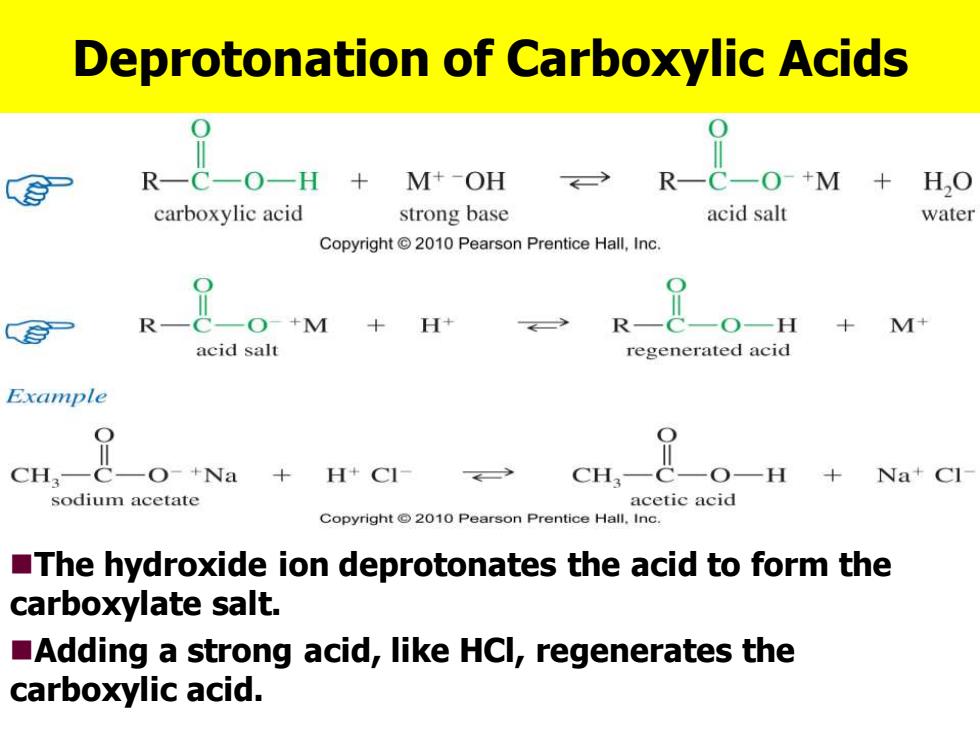
Deprotonation of Carboxylic Acids R一C一O+M H,O carboxylic acid strong base acid salt water Copyright 2010 Pearson Prentice Hall,Inc. 二O+M+ +M+ acid salt regenerated acid Example CH,— O-+Na +H+C1 cH, ,O- +Na+Cl sodium acetate acetic acid Copyright 2010 Pearson Prentice Hall.Inc. The hydroxide ion deprotonates the acid to form the carboxylate salt. Adding a strong acid,like HCl,regenerates the carboxylic acid.Deprotonation of Carboxylic Acids ◼The hydroxide ion deprotonates the acid to form the carboxylate salt. ◼Adding a strong acid, like HCl, regenerates the carboxylic acid