正在加载图片...
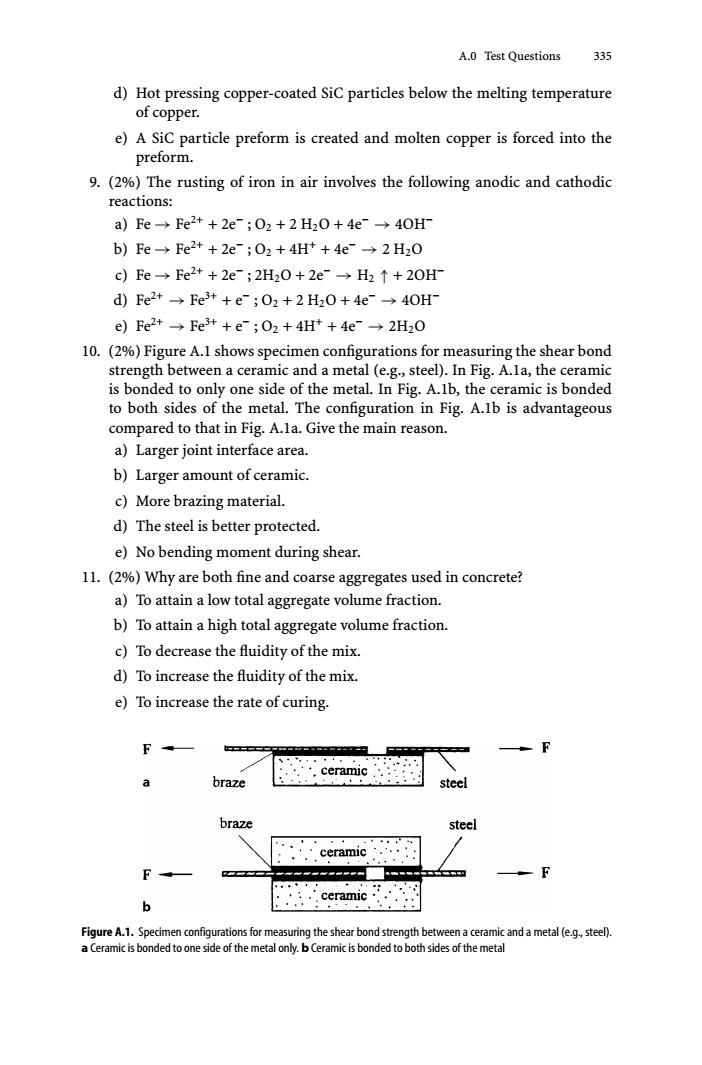
A.0 Test Questions 335 d)Hot pressing copper-coated SiC particles below the melting temperature of copper. e)A SiC particle preform is created and molten copper is forced into the preform. 9.(2%)The rusting of iron in air involves the following anodic and cathodic reactions: a)Fe→Fe2++2e;02+2H20+4e→40H b)Fe→Fe2++2e;02+4Ht+4e→2H20 c)Fe→Fe2++2e;2H20+2e→H2↑+20H d)Fe2+→Fe3t+e;02+2H20+4e→40H e)Fe2+→Fe3t+e;02+4Ht+4e→2H20 10.(2%)Figure A.1 shows specimen configurations for measuring the shear bond strength between a ceramic and a metal (e.g.,steel).In Fig.A.la,the ceramic is bonded to only one side of the metal.In Fig.A.lb,the ceramic is bonded to both sides of the metal.The configuration in Fig.A.1b is advantageous compared to that in Fig.A.la.Give the main reason. a)Larger joint interface area. b)Larger amount of ceramic. c)More brazing material. d)The steel is better protected. e)No bending moment during shear. 11.(2%)Why are both fine and coarse aggregates used in concrete? a)To attain a low total aggregate volume fraction. b)To attain a high total aggregate volume fraction. c)To decrease the fluidity of the mix. d)To increase the fluidity of the mix. e)To increase the rate of curing. ,··,ceramic a braze steel braze steel ceramic F b .ceramic Figure A.1.Specimen configurations for measuring the shear bond strength between a ceramic and a metal (e.g.,steel). a Ceramic is bonded to one side of the metal only.b Ceramic is bonded to both sides of the metalA.0 Test Questions 335 d) Hot pressing copper-coated SiC particles below the melting temperature of copper. e) A SiC particle preform is created and molten copper is forced into the preform. 9. (2%) The rusting of iron in air involves the following anodic and cathodic reactions: a) Fe → Fe2+ + 2e− ; O2 +2H2O + 4e− → 4OH− b) Fe → Fe2+ + 2e− ; O2 + 4H+ + 4e− → 2 H2O c) Fe → Fe2+ + 2e− ; 2H2O + 2e− → H2 ↑ + 2OH− d) Fe2+ → Fe3+ + e− ; O2 +2H2O + 4e− → 4OH− e) Fe2+ → Fe3+ + e− ; O2 + 4H+ + 4e− → 2H2O 10. (2%) Figure A.1 shows specimen configurations for measuring the shear bond strength between a ceramic and a metal (e.g., steel). In Fig. A.1a, the ceramic is bonded to only one side of the metal. In Fig. A.1b, the ceramic is bonded to both sides of the metal. The configuration in Fig. A.1b is advantageous compared to that in Fig. A.1a. Give the main reason. a) Larger joint interface area. b) Larger amount of ceramic. c) More brazing material. d) The steel is better protected. e) No bending moment during shear. 11. (2%) Why are both fine and coarse aggregates used in concrete? a) To attain a low total aggregate volume fraction. b) To attain a high total aggregate volume fraction. c) To decrease the fluidity of the mix. d) To increase the fluidity of the mix. e) To increase the rate of curing. Figure A.1. Specimen configurations for measuring the shear bond strength between a ceramic and a metal (e.g., steel). a Ceramic is bonded to one side of the metal only. b Ceramic is bonded to both sides of the metal