正在加载图片...
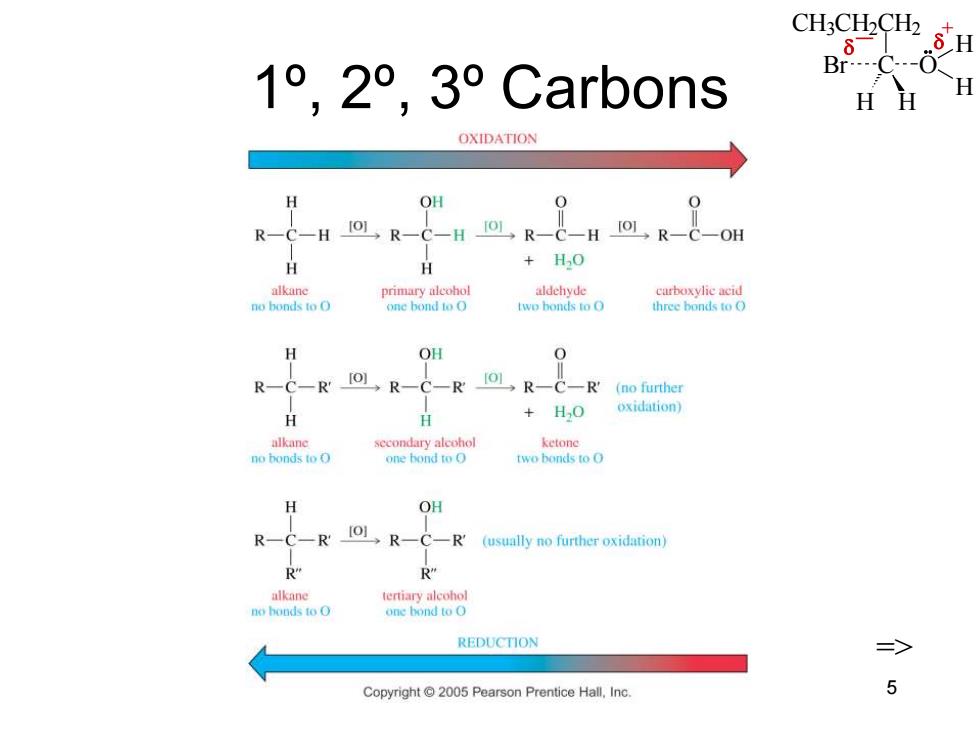
CH3CH2CH2 1°,2°,3°Carb0ns HH H OXIDATION H OH R-C-H.R-C-H IOL R-C-H [R-C-OH H H +H0 alkane primary alcohol aldehyde curboxylie acid no bonds to O one bond to O two bonds to O three bonds to O H OH R-C-R IOL R-C-R 101,R-C-R'(no further H +HO oxidation) alkane secondary alcohol ketone no bonds to O one hond to O two bonds to O H OH R-C-R R-C-R (usually no further oxidation) R" R” alkane tertiary alcohol no honds to O one hond to O REDUCTION 三> Copyright 2005 Pearson Prentice Hall,Inc 5CH3CH2CH2 C H H Br O H H _ + Chapter 11 5 1º, 2º, 3º Carbons =>