正在加载图片...
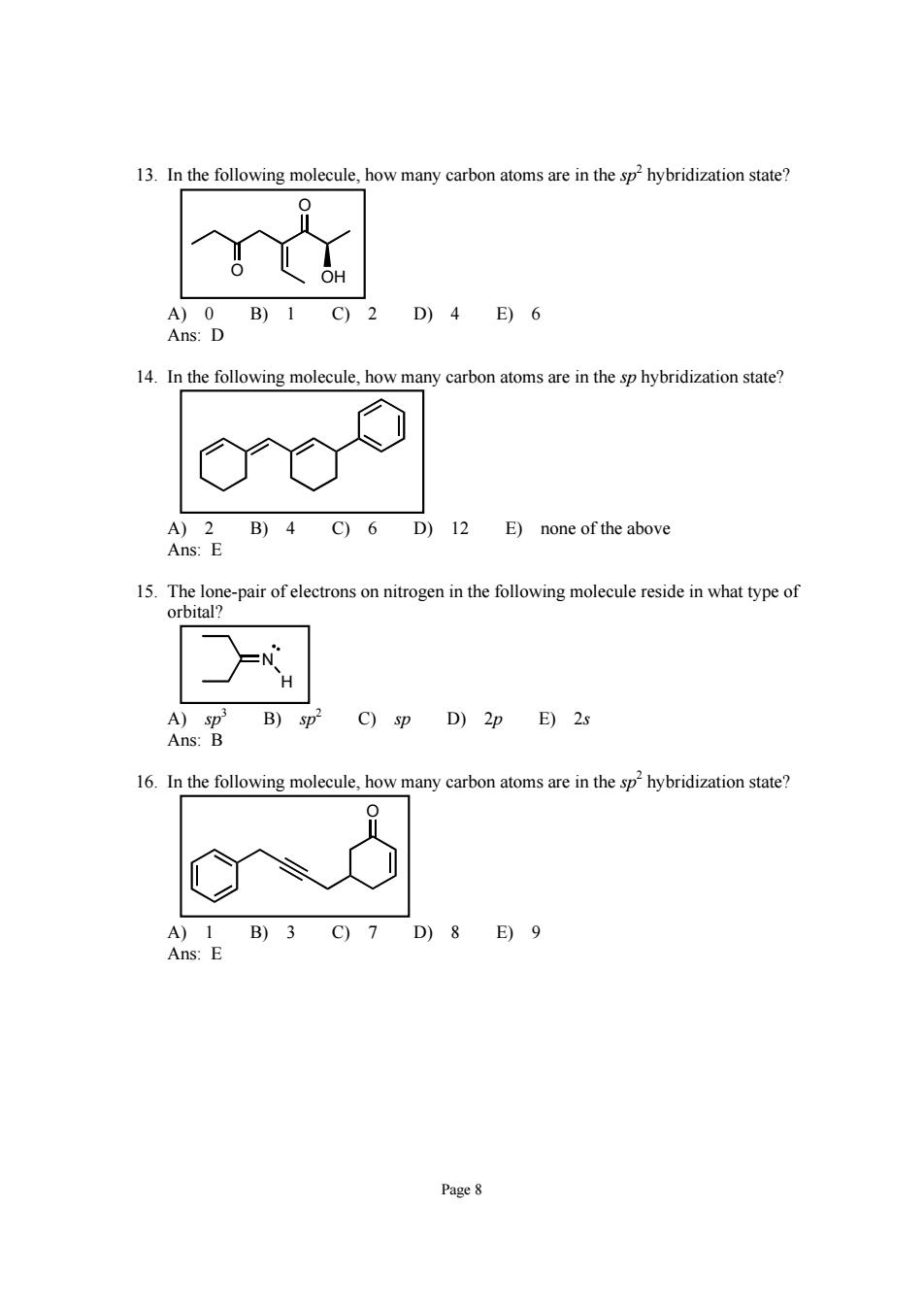
13.In the following molecule,how many carbon atoms are in the sp2hybridization state? A)0B)1C)2D)4E)6 Ans:D 14.In the following molecule,how many carbon atoms are in the sp hybridization state? B)4C)6 D)12 E)none of the above 15.The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? C)sp D)2p E)2s 16.In the following molecule how many carbon atoms are in the sphybridization state A)1B)3C)7D)8E)9 Ans:E Page 8 Page 8 13. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O O OH A) 0 B) 1 C) 2 D) 4 E) 6 Ans: D 14. In the following molecule, how many carbon atoms are in the sp hybridization state? A) 2 B) 4 C) 6 D) 12 E) none of the above Ans: E 15. The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? H N A) sp 3 B) sp 2 C) sp D) 2p E) 2s Ans: B 16. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O A) 1 B) 3 C) 7 D) 8 E) 9 Ans: E