正在加载图片...
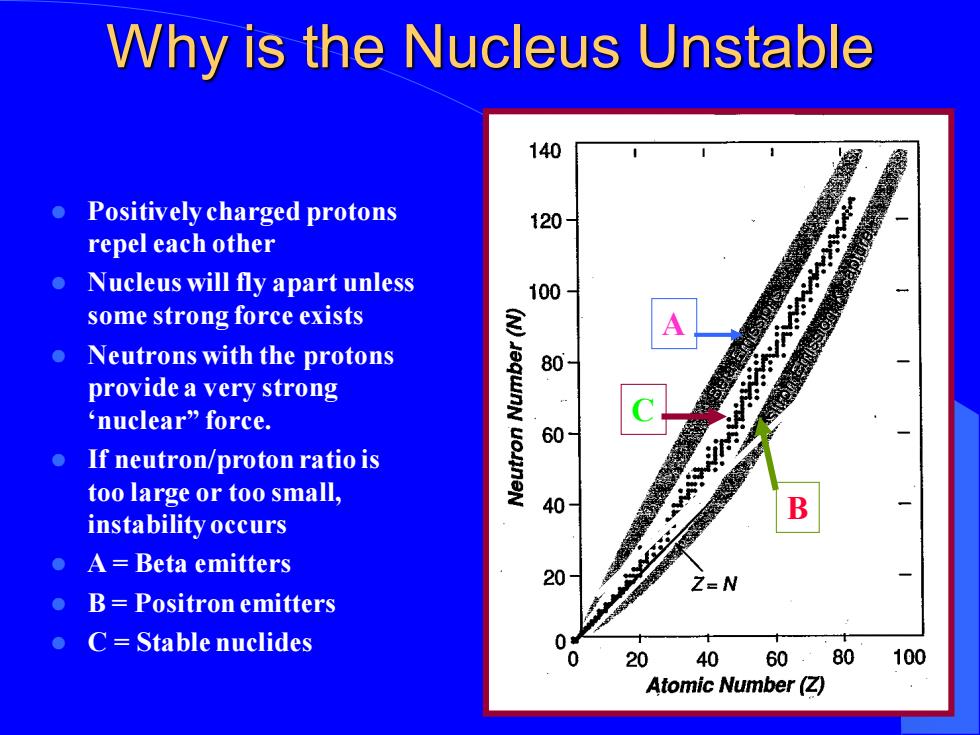
Why is the Nucleus Unstable 140 ● Positively charged protons 120 repel each other ● Nucleus will fly apart unless 100 some strong force exists Neutrons with the protons 80 provide a very strong 'nuclear”force. 60 If neutron/proton ratio is too large or too small, 40 B instability occurs A=Beta emitters 20 Z=N B=Positron emitters C=Stable nuclides 0 20 40 60.80100 Atomic Number(Z)Why is the Nucleus Unstable ⚫ Positively charged protons repel each other ⚫ Nucleus will fly apart unless some strong force exists ⚫ Neutrons with the protons provide a very strong ‘nuclear” force. ⚫ If neutron/proton ratio is too large or too small, instability occurs ⚫ A = Beta emitters ⚫ B = Positron emitters ⚫ C = Stable nuclides A B C