正在加载图片...
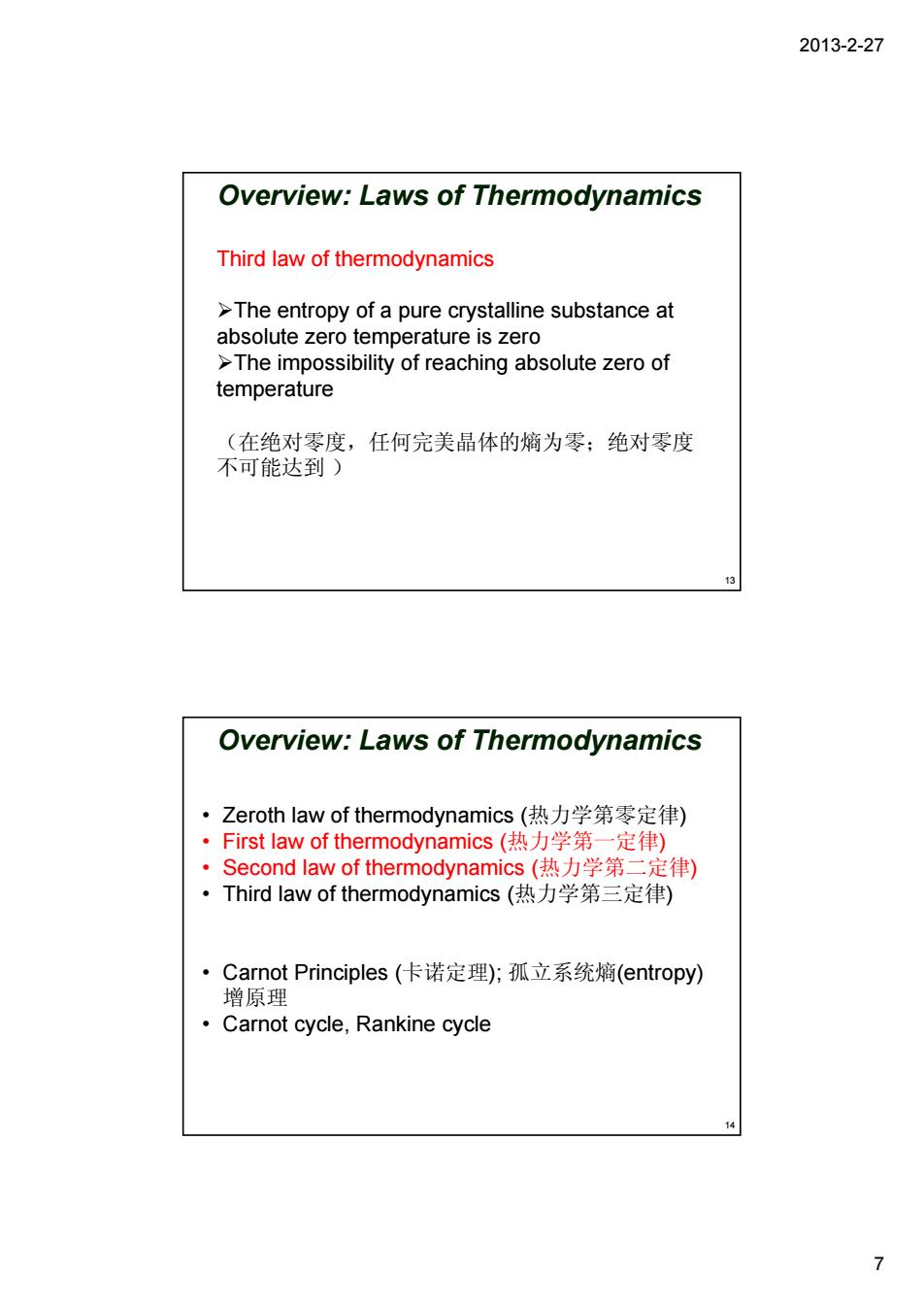
2013-2-27 Overview:Laws of Thermodynamics Third law of thermodynamics >The entropy of a pure crystalline substance at absolute zero temperature is zero >The impossibility of reaching absolute zero of temperature (在绝对零度,任何完美晶体的熵为零:绝对零度 不可能达到) Overview:Laws of Thermodynamics ·Zeroth law of thermodynamics(热力学第零定律) ·First law of thermodynamics(热力学第一定律) ·Second law of thermodynamics(热力学第二定律) ·Third law of thermodynamics(热力学第三定律) ·Camnot Principles(卡诺定理;孤立系统熵(entropy) 增原理 Carnot cycle,Rankine cycle 7 2013-2-27 7 Third law of thermodynamics ¾The entropy of a pure crystalline substance at absolute zero temperature is zero ¾The impossibility of reaching absolute zero of temperature (在绝对零度,任何完美晶体的熵为零;绝对零度 不可能达到 ) 13 Overview: Laws of Thermodynamics • Zeroth law of thermodynamics (热力学第零定律) • First law of thermodynamics (热力学第一定律) • Second law of thermodynamics (热力学第二定律) • Third law of thermodynamics (热力学第三定律) • Carnot Principles (卡诺定理); 孤立系统熵(entropy) 增原理 • Carnot cycle, Rankine cycle 14 Overview: Laws of Thermodynamics