正在加载图片...
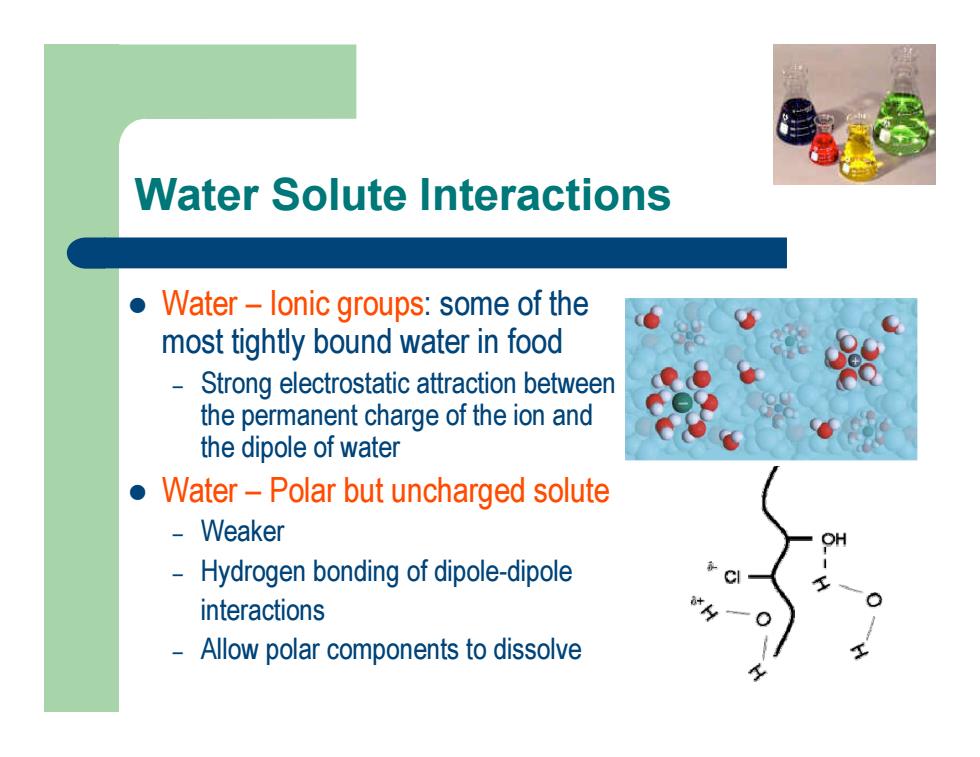
Water Solute Interactions l Water – Ionic groups: some of the most tightly bound water in food – Strong electrostatic attraction between the permanent charge of the ion and the dipole of water l Water – Polar but uncharged solute – Weaker – Hydrogen bonding of dipole-dipole interactions – Allow polar components to dissolveWater Solute Interactions l Water – Ionic groups: some of the most tightly bound water in food – Strong electrostatic attraction between the permanent charge of the ion and the dipole of water l Water – Polar but uncharged solute – Weaker – Hydrogen bonding of dipole-dipole interactions – Allow polar components to dissolve