正在加载图片...
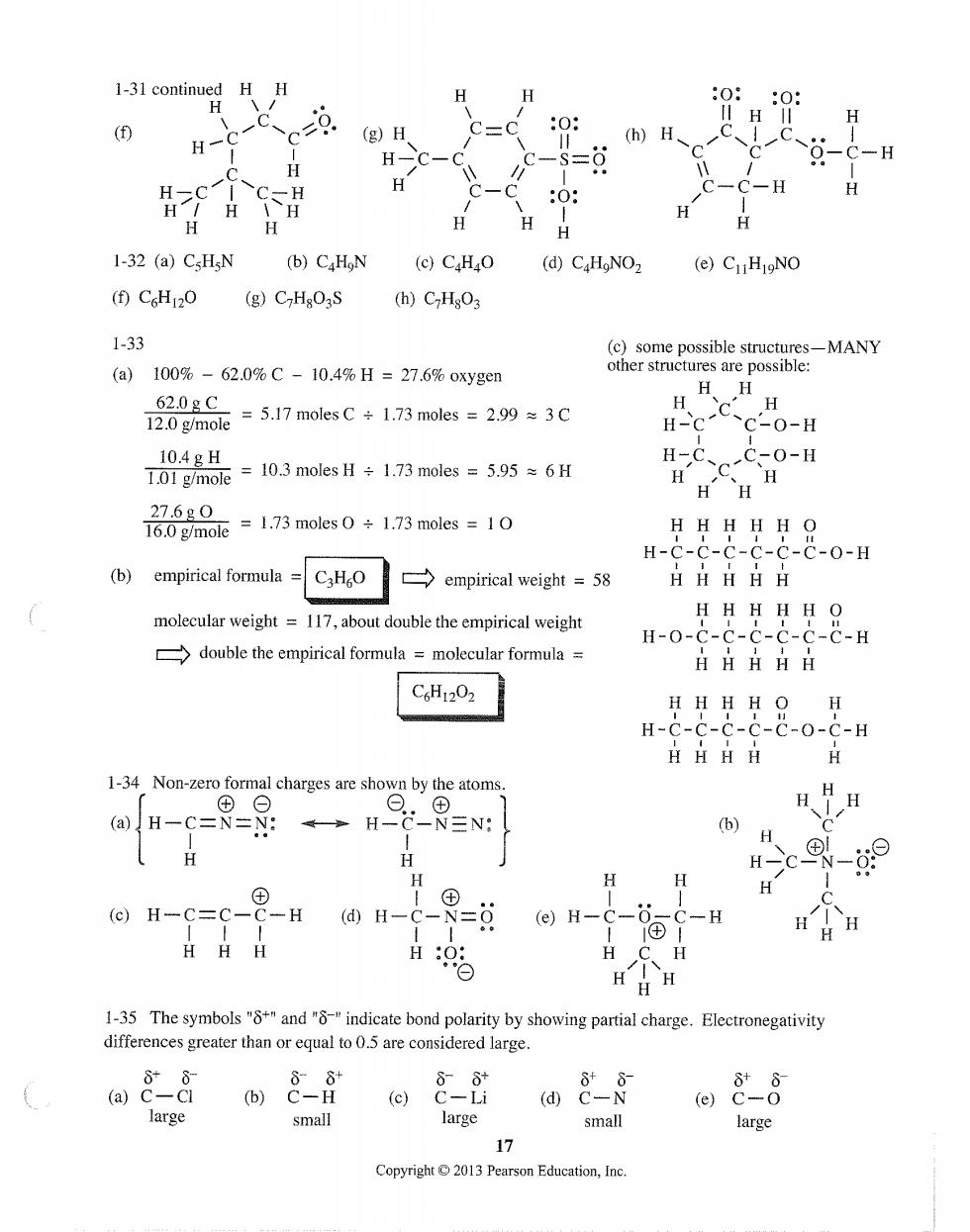
1-31 continued HH (g)H 1-32 (a)CsHN (b)CaHoN (e)C.HO (d)C.HNOz (e)CuHINO (0CH120 (g)CHgO3S (h)CzHsO3 1-33 (c)some possible structure es-MANY (a)100%-62.0%C-10.4%H=27.6%oxygen other structures are possible: 12.0mole 5.17 moles C 1.73 moles 2.99 =3C H-c-c C-0-H 10.4gH 1.01 g/mole ,=10.3 moles H÷1.73 moles=5.95≈6H 27.6g0 16.0 g/mole 1.73 moles O+1.73 moles 10 HHHHH O H-C-C-C-C-C-C-0-H (b)empirical formula=CH empirical weight=58 HHHHH molecular weight =117,about double the empirical weight HHHHH O H-0-C-C-C-C-C-C-H double the empirical formula =molecular formula HHHHH C6H1202 HHHH O H H-C-C-C-C-C-0-C-H HHHH H 1-34 Non-zero formal charges are shown by the atoms. (@)H-C=N-N: H9-9N H c9-9 H H (c)H-C=C- -H @H-C-8=过 (e)H- h9 1④ -H H H 1-35 The symbols"andindicate bond polarity by showing partial charge.Electronegativity differences greater than or equal to5 are considered large. 68 (b)C-H @88 large small large small large 17 Copyright013 Pearson Education,Inc