正在加载图片...
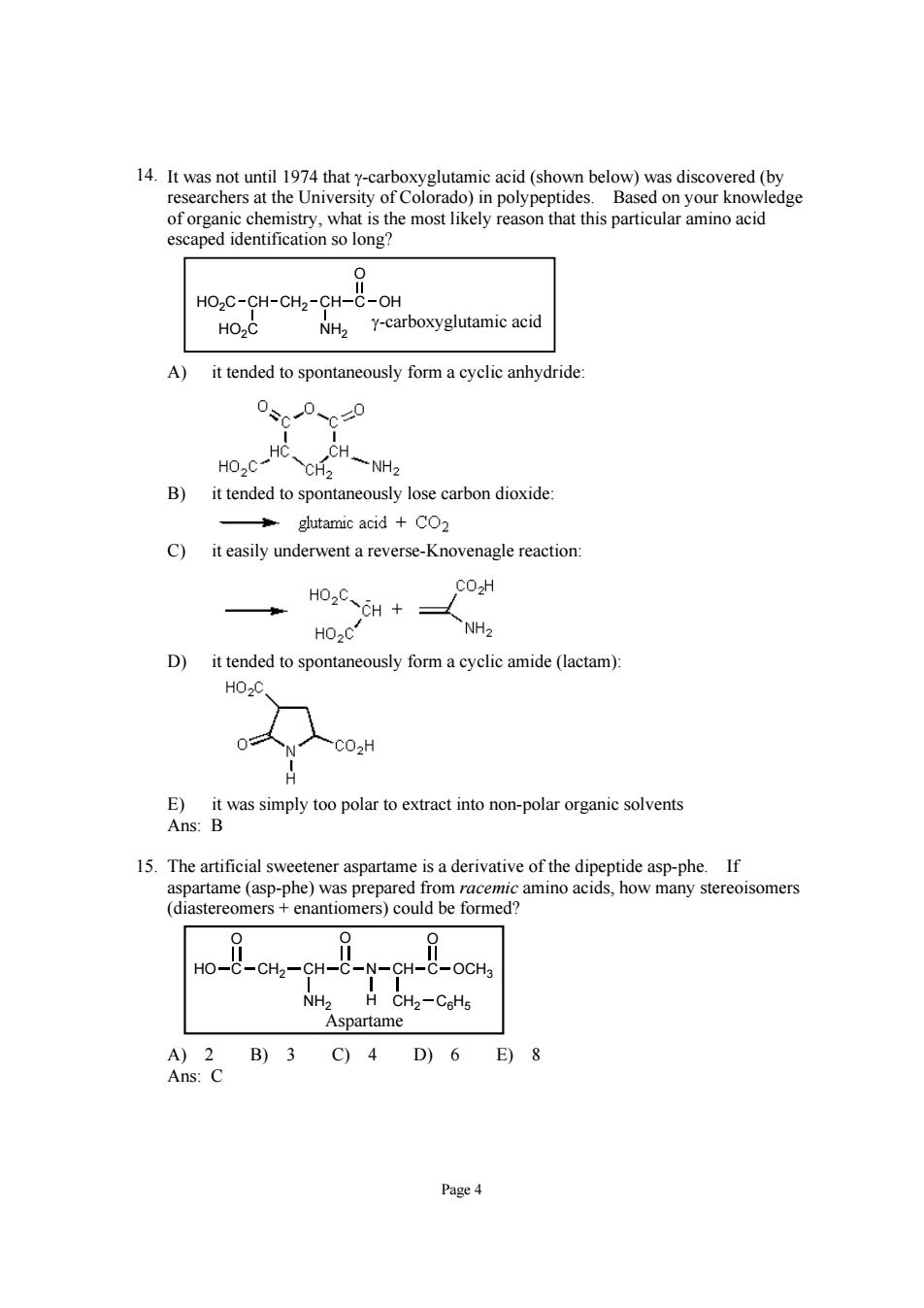
researchers ledge cation so long HO2C-CH-CH2-CH-C-OH HO2C NH2 Y-carboxyglutamic acid A) it tended to spontaneously form a cyclic anhydride 0、0、0 B)it tended to spontaneously lose carbon dioxide glutamic acid +CO2 C)it easily underwent a reverse-Knovenagle reaction HO.CCH+ CO2H HO2C D)it tended to spontaneously form a cyclic amide(lactam) HO.O 0 CO2H E)it was simply too polar to extract into non-polar organic solvents Ans:B 15.The artificial sweetener aspartame is a derivative of the dipeptide asp-phe.If aspartame(asp-phe)was prepared from racemic amino acids,how many stereoisomers (diastereomers enantiomers)could be formed? Ho--CH-CH- -C-OCHa Aspartame A)2B)3C)4D)6E)8 Ans:C Page4 Page 4 14. It was not until 1974 that γ-carboxyglutamic acid (shown below) was discovered (by researchers at the University of Colorado) in polypeptides. Based on your knowledge of organic chemistry, what is the most likely reason that this particular amino acid escaped identification so long? CH CH2 CH C OH O HO2C HO2C NH2 γ-carboxyglutamic acid A) it tended to spontaneously form a cyclic anhydride: B) it tended to spontaneously lose carbon dioxide: C) it easily underwent a reverse-Knovenagle reaction: D) it tended to spontaneously form a cyclic amide (lactam): E) it was simply too polar to extract into non-polar organic solvents Ans: B 15. The artificial sweetener aspartame is a derivative of the dipeptide asp-phe. If aspartame (asp-phe) was prepared from racemic amino acids, how many stereoisomers (diastereomers + enantiomers) could be formed? HO C O CH2 CH NH2 C O N CH H CH2 C6H5 C O OCH3 Aspartame A) 2 B) 3 C) 4 D) 6 E) 8 Ans: C