正在加载图片...
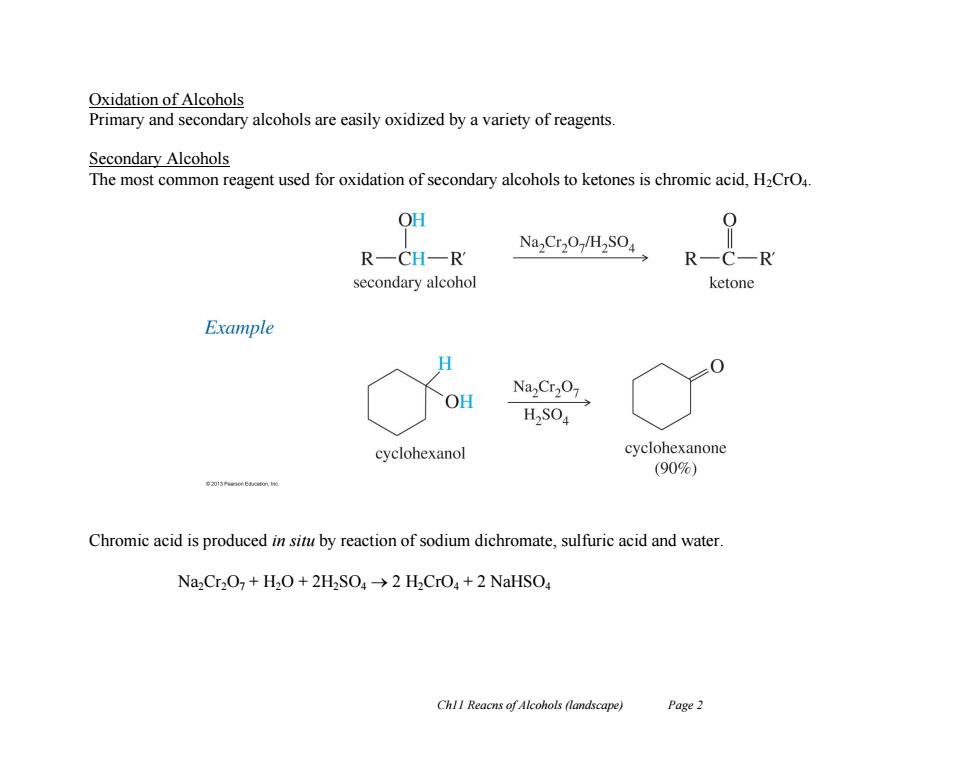
Oxidation of Alcohols Primary and secondary alcohols are easily oxidized by a variety of reagents Secondary Alcohols The most common reagent used for oxidation of secondary alcohols to ketones is chromic acid,H2CrO4. OH Na,Cr2O-/H2SO4 RCHR R C-R secondary alcohol ketone Example H NaCr207 OH H2SO4 cyclohexanol cyclohexanone (90%) 中1 Chromic acid is produced in situ by reaction of sodium dichromate,sulfuric acid and water. Na,Cr2O7+H2O+2H2SO>2 H2CrO+2 NaHSO Ch!I Reacns of Alcohols (landscape) Page 2 Ch11 Reacns of Alcohols (landscape) Page 2 Oxidation of Alcohols Primary and secondary alcohols are easily oxidized by a variety of reagents. Secondary Alcohols The most common reagent used for oxidation of secondary alcohols to ketones is chromic acid, H2CrO4. Chromic acid is produced in situ by reaction of sodium dichromate, sulfuric acid and water. Na2Cr2O7 + H2O + 2H2SO4 2 H2CrO4 + 2 NaHSO4