正在加载图片...
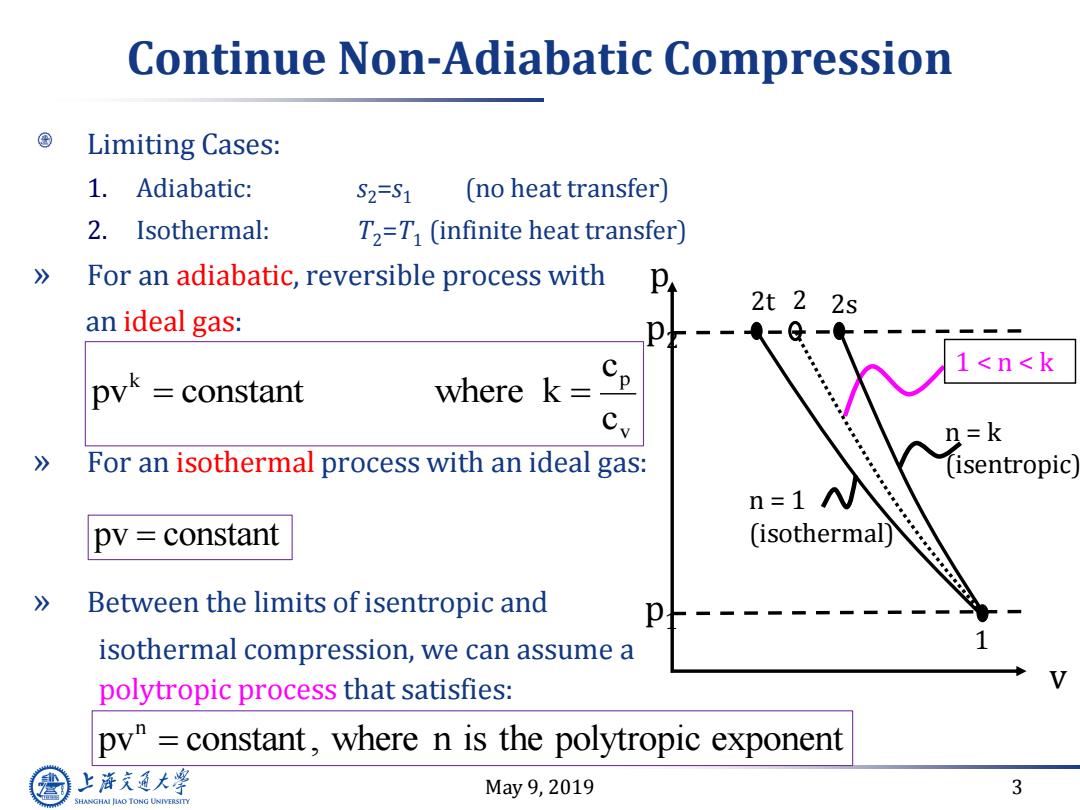
Continue Non-Adiabatic Compression Limiting Cases: 1.Adiabatic: S2=S1 (no heat transfer) 2.Isothermal: T2=T (infinite heat transfer) 》 For an adiabatic,reversible process with 2t22s an ideal gas: a-@ 1<n<k pyk =constant where k= 2=k 》 For an isothermal process with an ideal gas: (isentropic n=1 N pv constant (isothermal) 》 Between the limits of isentropic and isothermal compression,we can assume a polytropic process that satisfies: py"constant,where n is the polytropic exponent 上游究通大粤 May9,2019 3 SHANGHAI JLAO TONG UNIVERSITYMay 9, 2019 3 Continue Non-Adiabatic Compression Limiting Cases: 1. Adiabatic: s2=s1 (no heat transfer) 2. Isothermal: T2=T1 (infinite heat transfer) » For an adiabatic, reversible process with an ideal gas: » For an isothermal process with an ideal gas: » Between the limits of isentropic and isothermal compression, we can assume a polytropic process that satisfies: k p v c pv constant where k c pv constant n pv constant , where n is the polytropic exponen t v 1 2s p p2 p1 1 < n < k 2t 2 n = k (isentropic) n = 1 (isothermal)