正在加载图片...
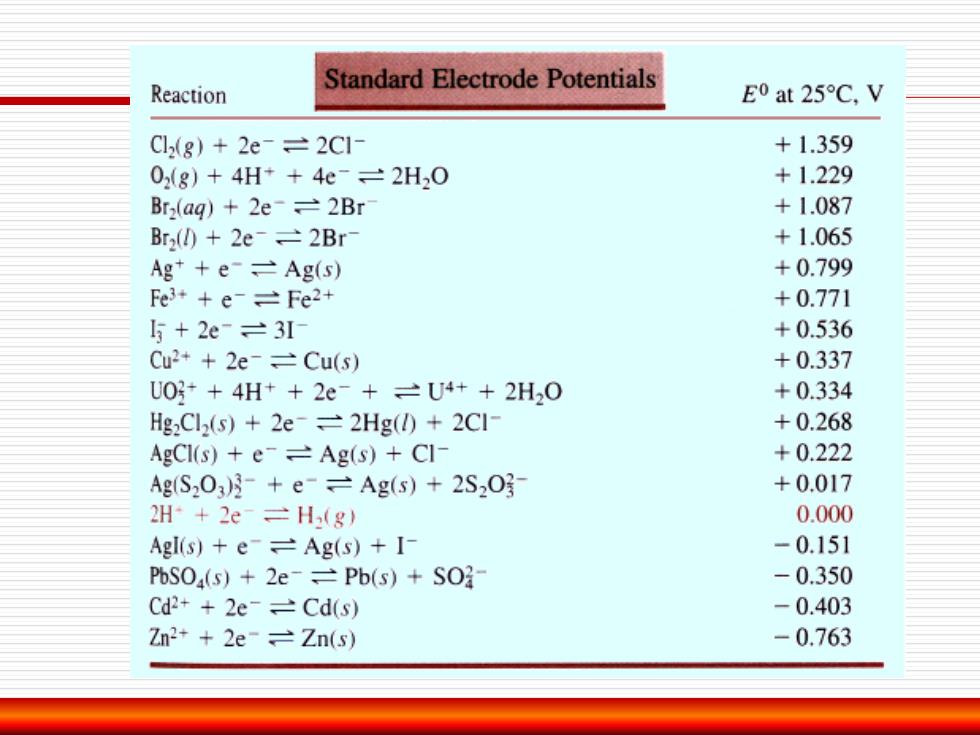
Standard Electrode Potentials Reaction EO at 25C,V Cl(8)+2e-=2CI- +1.359 0(8)+4H++4e-=2H2O +1.229 Bra(ag)+2e-2Br +1.087 Br2()2e-=2Br- +1.065 Ag +e-=Ag(s) +0.799 Fe3++e-=Fe2+ +0.771 5+2e=3I +0.536 Cu2+2e-=Cu(s) +0.337 U0g++4H++2e-+=U4++2H20 +0.334 Hg2Cl2(s)+2e-=2Hg(l)+2CI- +0.268 AgCl(s)+eAg(s)+Cl- +0.222 AgS203)月+e-=Ag(s)+2S203 +0.017 2H+2e=H(g) 0.000 Agl(s)e-=Ag(s)+I- -0.151 PbSOa(s)2e-=Pb(s)+SO- -0.350 Cd2+2e-=Cd(s) -0.403 Zn2+2e-Zn(s) -0.763