正在加载图片...
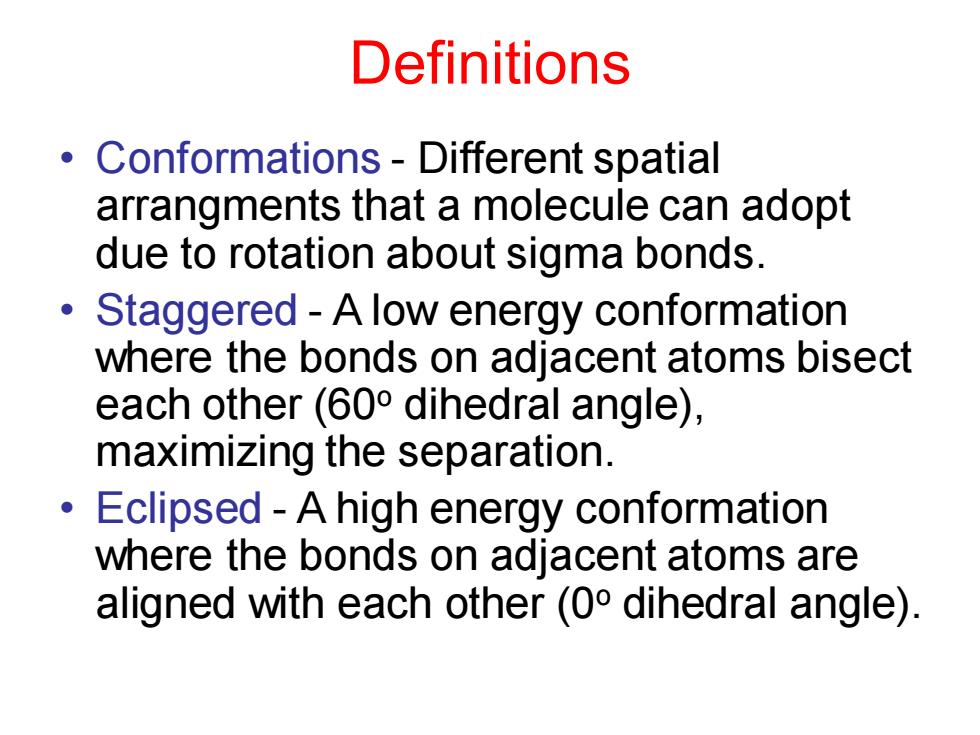
Definitions Conformations-Different spatial arrangments that a molecule can adopt due to rotation about sigma bonds. ● Staggered-A low energy conformation where the bonds on adjacent atoms bisect each other(600 dihedral angle), maximizing the separation. Eclipsed-A high energy conformation ● where the bonds on adjacent atoms are aligned with each other(0 dihedral angle). Definitions • Conformations - Different spatial arrangments that a molecule can adopt due to rotation about sigma bonds. • Staggered - A low energy conformation where the bonds on adjacent atoms bisect each other (60o dihedral angle), maximizing the separation. • Eclipsed - A high energy conformation where the bonds on adjacent atoms are aligned with each other (0o dihedral angle)