正在加载图片...
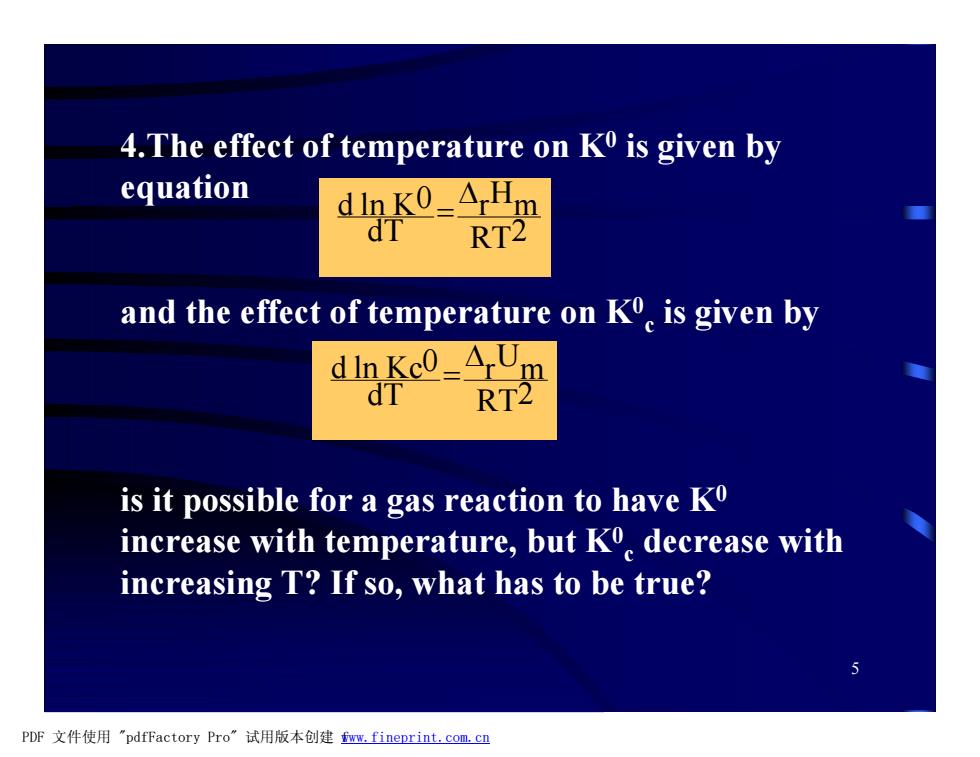
4.The effect of temperature on K0 is given by equation dink0=ArHm dT RT2 and the effect of temperature on Ko is given by dIn Kc0=ArUm dT RT2 is it possible for a gas reaction to have Ko increase with temperature,but K decrease with increasing T?If so,what has to be true? PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn5 4.The effect of temperature on K0 is given by equation and the effect of temperature on K0 c is given by is it possible for a gas reaction to have K0 increase with temperature, but K0 c decrease with increasing T? If so, what has to be true? RT2 r Hm dT d ln K0 D = RT2 r Um dT d ln Kc0 D = PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn