
Chemical equilibrium Tutorial Lecture PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
1 Chemical equilibrium Tutorial Lecture PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn f

1.For a chemical reaction, InK=a+b/T+C/T2.Derive the corresponding expressions to calculate△,Gm,△rlm,A,SOm and△,Cp,m PDF文件使用"pdfFactory Pro”试用版本创建mm,fineprint..com,cn
2 1. For a chemical reaction, lnK=a+b/T+c/T2 . Derive the corresponding expressions to calculate DrG0 m ,DrH0 m ,DrS 0 m and DrCp,m PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn

2.Assume that the following reaction is in equilibrium at 1000K: 3C(graphite)+2H2O(g)=CH4(g)+2CO(g) △,H0m(1000K=l82 kJmol-'.(a)What will be the effect on the equilibrium composition of raising the temperature at a total pressure of 1 bar?(b)What will be the effect of raising the pressure to 5 bar? OWhat will be the effect of adding nitrogen at a constant pressure of 1 bar? PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
3 2. Assume that the following reaction is in equilibrium at 1000K: 3C(graphite)+2H2O(g)=CH4 (g)+2CO(g) DrH0 m (1000K)=182kJmol-1. (a) What will be the effect on the equilibrium composition of raising the temperature at a total pressure of 1 bar?(b) What will be the effect of raising the pressure to 5 bar? ©What will be the effect of adding nitrogen at a constant pressure of 1 bar? PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

3.The following exothermic reaction is at equilibrium at 500K and 10 bar: CO(g)+2H2(g)=CH3OH(g) Assuming that the gases are ideal,what will happen to the amount of methanol at equilibrium when (a)the temperature is raised,(b)the pressure is increased,o an inert gas is pumped in at constant volume,(d)an inert gas is pumped in at constant pressure,and (e)hydrogen gas is added at constant pressure? PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint..com,cn
4 3.The following exothermic reaction is at equilibrium at 500K and 10 bar: CO(g)+2H2 (g)=CH3OH(g) Assuming that the gases are ideal, what will happen to the amount of methanol at equilibrium when (a) the temperature is raised,(b) the pressure is increased,© an inert gas is pumped in at constant volume,(d) an inert gas is pumped in at constant pressure, and (e) hydrogen gas is added at constant pressure? PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f
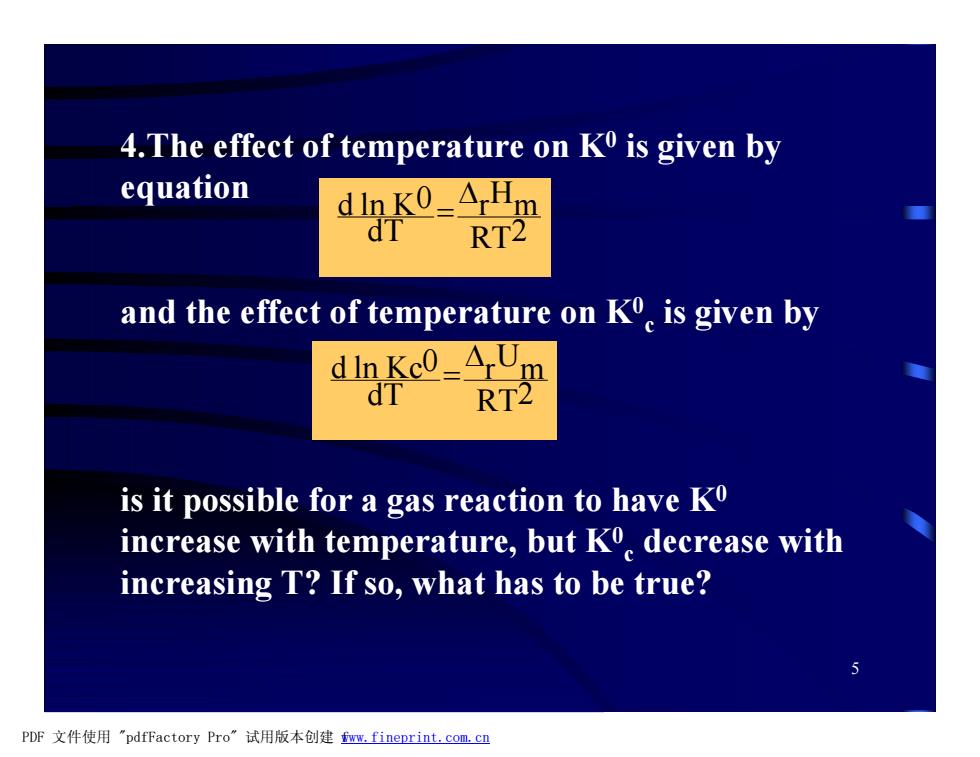
4.The effect of temperature on K0 is given by equation dink0=ArHm dT RT2 and the effect of temperature on Ko is given by dIn Kc0=ArUm dT RT2 is it possible for a gas reaction to have Ko increase with temperature,but K decrease with increasing T?If so,what has to be true? PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
5 4.The effect of temperature on K0 is given by equation and the effect of temperature on K0 c is given by is it possible for a gas reaction to have K0 increase with temperature, but K0 c decrease with increasing T? If so, what has to be true? RT2 r Hm dT d ln K0 D = RT2 r Um dT d ln Kc0 D = PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn