
Experiment Electrochemical Determination of AG,AH,and AS Objectives .To determine thermodynamic properties using electrochemical techniques Introduction odynamics is with the energy changes that ccur in chemical and physica processes.Several fundamental laws summarize these energy changes The first law of thermodynamics states that energy is conserved.that is.it can not be created or destroyed.Energy can be converted from one form to another,but the total remains constant.In chemical reactions,rearrangements of atoms and electrons occur,leading to new which interact with their su roundings This changes the chemical potentil of the system and thus hea △H=Heu-H The second law of thermodynamies relates to the spontaneous nature of a chemical process. It has been established through observation that proces sses that are s可 ontaneous in one direc ion are no sponta direction.For insta nce.if you drop a b hook on the floor it does no spontaneously move back into your hand.This fact applies to chemical as well as physical systems Spontaneity is associated with an increase in randomness or disorder of a system. Thermodynamically,this is expressed by a quantity called entropy.S.the change in entropy of a system is expressed as △S=Saa-Sna The free energy,G,interrelates the enthalpy and entropy of a system.For a process occurring at constant temperature and pressure,the change in free energy of a system is expressed by △G=△H-T△S The sign of AG relates to the spontaneity of the reaction 1.If AG is negative,the reaction is spontaneous in the forward direction. 2.If AG is zero,the reaction is at equilibrium. 3.If AG is positive.the reaction is spontaneous in the reverse direction. The quantitiesG.AH and S depend ony on the initial and final states of a sys and ot on the pathway of the change.called state are said tob path independent The investigation of electrochemical reactions offers a simple and direct method for measuring thermodynamic values,because the quantity of electrical energy produced or consumed during an electrochemical reaction can be measured very accurately.The free energy change,AG △G=-nFE Where nis the number of moles of electrons transferred in the reaction per mole of reactant. and Fis Faraday's constant. PDF文件使用"pdfFactory Pro”试用版本创建wnw,fineprint,com,cn
Experiment Electrochemical Determination of ΔG, ΔH, and ΔS Objectives • To determine thermodynamic properties using electrochemical techniques. Introduction Thermodynamics is concerned with the energy changes that occur in chemical and physical processes. Several fundamental laws summarize these energy changes. The first law of thermodynamics states that energy is conserved, that is, it can not be created or destroyed. Energy can be converted from one form to another, but the total remains constant. In chemical reactions, rearrangements of atoms and electrons occur, leading to new species, which interact with their surroundings. This changes the chemical potential of the system, and thus heat is evolved or absorbed. This heat energy is usually expressed as enthalpy, DH H H final initial = - The second law of thermodynamics relates to the spontaneous nature of a chemical process. It has been established through observation that processes that are spontaneous in one direction are not spontaneous in reverse direction. For instance, if you drop a book on the floor, it does not spontaneously move back into your hand. This fact applies to chemical as well as physical systems. Spontaneity is associated with an increase in randomness or disorder of a system. Thermodynamically, this is expressed by a quantity called entropy, S. the change in entropy of a system is expressed as final initial DS = - S S The free energy, G, interrelates the enthalpy and entropy of a system. For a process occurring at constant temperature and pressure, the change in free energy of a system is expressed by DG = DH - DT S (1) The sign of DG relates to the spontaneity of the reaction 1. If DG is negative, the reaction is spontaneous in the forward direction. 2. If DG is zero, the reaction is at equilibrium. 3. If DG is positive, the reaction is spontaneous in the reverse direction. The quantitiesDG , DH and DS depend only on the initial and final states of a system and not on the pathway of the change. Such quantities are called state functions and are said to be path independent. The investigation of electrochemical reactions offers a simple and direct method for measuring thermodynamic values, because the quantity of electrical energy produced or consumed during an electrochemical reaction can be measured very accurately. The free energy change, DG , of an electrochemical reaction is related to the voltage, E , of the electrochemical cell: DG = -nFE (2) Where n is the number of moles of electrons transferred in the reaction per mole of reactant, and F is Faraday’s constant. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Ifvoltage measurements of the cell are made at different temperatures,the change in entropy. AS. of the system can be expressed at constant pressure as -4S=d4G) (3) dT Substitution of Equation 2 into Equation I gives -AS=-d(nFE) -AS=nF dE (4) Faraday's constant (is the electrical charge on 1 mol of electrons 96 500 I Vmol E is express olts and the t em perature in K.AS an he expressed as calKmol(1 calorie =4.184J).Note that the quantity dE/dT in Equation 4 is a slope-the change in the electrochemical voltage with respect to temperature.Thus,a plot of E(y axis)versus 7(x axis) will have an instantaneous slope(tangent)of dE/dT at any temperature T. In this experiment,the electrochemical cell will consist of the Zn/nand Cu/Cuhalfcells. connected by asalt bridge.The half-cell reactions are Zn Zn*+2e (oxidation at the anode) Cu"+2e- Cu (reduction at the cathode) Zn+Cu2 Zn2+Cu (overall cell reaction) Tables of half-cell potentials can be found in Appendix.It should be noted that the potentials given in these tables are of E that is.the standard potentials.Real potentials.E.depend on temperature,concentraionof irons in solution etcand are obtained from the Nernst quation nF In most cases,concentration can be used in place of activity without incurring too large of an error. the various constants are collected,and the expression is evaluated at 25C,resulting in a simplified version of the Nernst equation: n [reac tan ts] When the concentrations of the Znand Cusolutions are equal,the log part of the Nernst deredrdnd be 1.100V the cell described.(Do btai tree energy △G,may be △G=-nFE ( In this experiment,the cell voltage will be measured at three or more temperatures.If several temperatures are used,a plot of E versus Tyields the value of dE/dT from the instantaneous slope of the line,as previously discussed.Substitution of this slope into Equation 4 gives AS.The value PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
If voltage measurements of the cell are made at different temperatures, the change in entropy, DS , of the system can be expressed at constant pressure as d G ( ) S dT D -D = (3) Substitution of Equation 2 into Equation 1 gives d( ) nFE S dT - -D = or dE S nF dT -D = (4) Faraday’s constant (F) is the electrical charge on 1 mol of electrons, 96,500 J V-1mol. E is expresses in volts and the temperature in K. DS can be expressed as J V-1mol-1 or cal K-1mol-1 (1 calorie = 4.184J). Note that the quantity dE / dT in Equation 4 is a slope-the change in the electrochemical voltage with respect to temperature. Thus, a plot of E (y axis) versus T (x axis) will have an instantaneous slope (tangent) of dE / dT at any temperature T. In this experiment, the electrochemical cell will consist of the Zn/Zn2+ and Cu/Cu2+ half-cells, connected by a salt bridge. The half-cell reactions are 2+ Zn Zn 2e- + (oxidation at the anode) 2+ Cu +2e Cu - (reduction at the cathode) __________________________________________________ 2+ 2+ Zn+Cu Zn +Cu (overall cell reaction) Tables of half-cell potentials can be found in Appendix. It should be noted that the potentials given in these tables are of o E , that is, the standard potentials. Real potentials, E, depend on temperature, concentration of irons in solution, etc., and are obtained from the Nernst equation: products reac tan ts ln o RT a E E nF a = - In most cases, concentration can be used in place of activity without incurring too large of an error, the various constants are collected, and the expression is evaluated at 25℃, resulting in a simplified version of the Nernst equation: 0.059 [products] ln [reac tan ts] o E E n = - When the concentrations of the Zn2+ and Cu2+ solutions are equal, the log part of the Nernst equation reduces to zero. Under these conditions, the standard voltage, o E , and will be 1.100V for the cell described. (Do you experimentally obtain this value for E?). The free energy, ΔG, may be obtained from the voltage by the equation DG = -nFE (5) In this experiment, the cell voltage will be measured at three or more temperatures. If several temperatures are used, a plot of E versus T yields the value of dE/dT from the instantaneous slope of the line, as previously discussed. Substitution of this slope into Equation 4 gives ΔS. The value PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
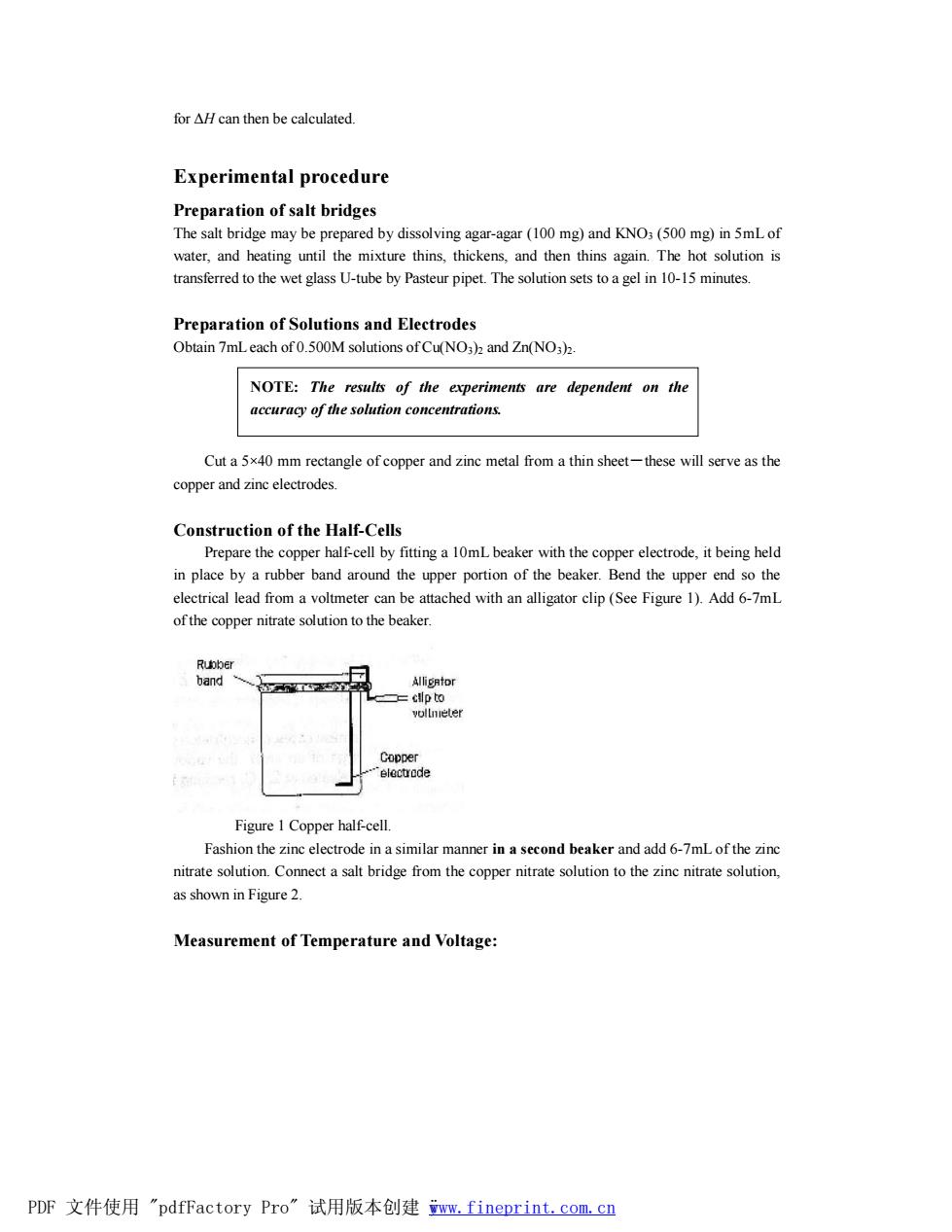
for H can then be calculated. Experimental procedure Preparation of salt bridges The salt bridge may be prepared by dissolving agar-agar(100 mg)and KNO:(500 mg)in 5mLof water,and heating until th mixture thins thickens and then thins again.The hot solution is transferred to the Preparation of Solutions and Electrodes Obtain 7mLeach of0.500M solutions of Cu(NO3)and Zn(NO3)2. NOTE:The results of the experiments are dependent on the accuracy of the solution concentrations. Cut a5x40 mm rectangle of copper and zinc metal from a thin sheet-these will serve as the copper and zinc electrodes. Construction of the Half-Cells Prepare the copper half-cell by fitting a 10mL beaker with the copper electrode.it being held in place by a rubber band around the upper portion of the beaker.Bend the upper end so the electrical lead from a voltmeter can be attached with an alligator clip(See Figure 1).Add 6-7mL ofthe copper nitrate solution to the beaker. Figure I Copper half-cell. Fashion the zinc electrode in a similar manner in a second beaker and add 6-7mLof the zinc nitratesoton Connect a salt bridge from the copper nitratesotionto the zinc nitratesolution. as shown in Figure 2. Measurement of Temperature and Voltage: PDF文件使用"pdfFactory Pro”试用版本创建n,fineprint.com,cn
for ΔH can then be calculated. Experimental procedure Preparation of salt bridges The salt bridge may be prepared by dissolving agar-agar (100 mg) and KNO3 (500 mg) in 5mL of water, and heating until the mixture thins, thickens, and then thins again. The hot solution is transferred to the wet glass U-tube by Pasteur pipet. The solution sets to a gel in 10-15 minutes. Preparation of Solutions and Electrodes Obtain 7mL each of 0.500M solutions of Cu(NO3)2 and Zn(NO3)2. Cut a 5×40 mm rectangle of copper and zinc metal from a thin sheet-these will serve as the copper and zinc electrodes. Construction of the Half-Cells Prepare the copper half-cell by fitting a 10mL beaker with the copper electrode, it being held in place by a rubber band around the upper portion of the beaker. Bend the upper end so the electrical lead from a voltmeter can be attached with an alligator clip (See Figure 1). Add 6-7mL of the copper nitrate solution to the beaker. Figure 1 Copper half-cell. Fashion the zinc electrode in a similar manner in a second beaker and add 6-7mL of the zinc nitrate solution. Connect a salt bridge from the copper nitrate solution to the zinc nitrate solution, as shown in Figure 2. Measurement of Temperature and Voltage: NOTE: The results of the experiments are dependent on the accuracy of the solution concentrations. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn
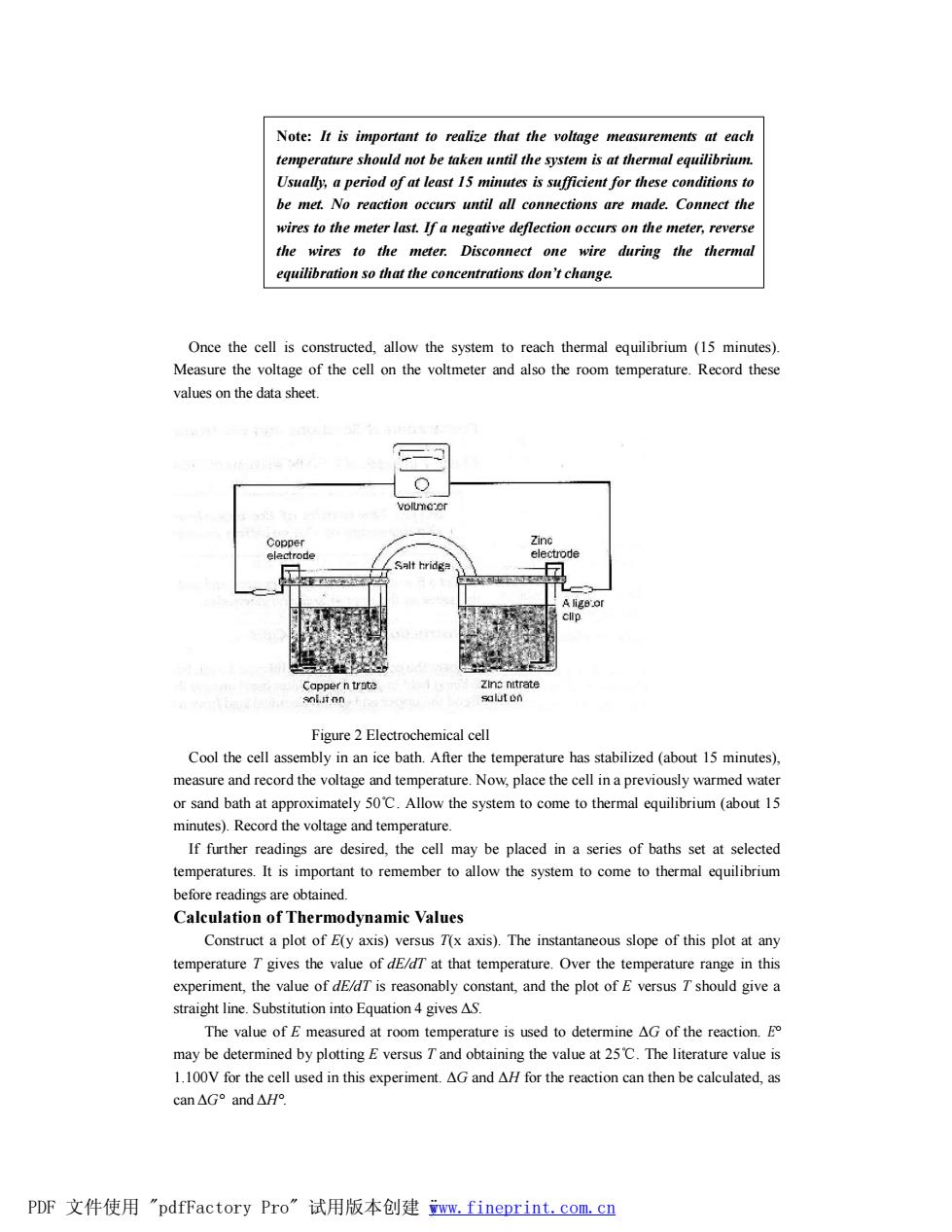
Note:It is important to realize that the voltage measurements at each ent for be met. o reaction occurs until all connections are made.Comnect the wires to the meter last.If a negative deflection occurs on the meter,reverse the wires to the meter.Disconnect one wire during the thermal equilibration so that the concentrations don't change. Once the cell is constructed,allow the system to reach thermal equilibrium(15 minutes). Measure the voltage of the cell on the volt mete also the room temperature Recor these values on the data shee Figure 2 Electrochemical ce Cool the cell assembly inn ice bath. After th e temperature has stabilized(about 15 minutes) measure and record the voltage and temperature.Now,place the cell in a previously warmed wate or sand bath at approximately 50C.Allow the system to come to thermal equilibrium (about 15 minutes).Record the voltage and temperature. If further readings are desired.the cell may be placed in a series of baths set at selected temperatures.Itis imporant to remember to allow the system to come to the quilibr before readings are obtained Calculation of Thermodynamic Values Construct a plot of E(y axis)versus T(x axis).The instantaneous slope of this plot at any temperature T gives the value of de/dT at that temperature.Over the temperature range in this expe ment the value of dE is reasonably constant,and the plot of E versus Tshould give The value of Emeasured at room temperature is used to determine AG of the reaction.g may be determined by plotting E versus Tand obtaining the value at 25C.The literature value is 1.100V for the cell used in this experiment.AG and AH for the reaction can then be calculated,as can△G°and△He PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
Once the cell is constructed, allow the system to reach thermal equilibrium (15 minutes). Measure the voltage of the cell on the voltmeter and also the room temperature. Record these values on the data sheet. Figure 2 Electrochemical cell Cool the cell assembly in an ice bath. After the temperature has stabilized (about 15 minutes), measure and record the voltage and temperature. Now, place the cell in a previously warmed water or sand bath at approximately 50℃. Allow the system to come to thermal equilibrium (about 15 minutes). Record the voltage and temperature. If further readings are desired, the cell may be placed in a series of baths set at selected temperatures. It is important to remember to allow the system to come to thermal equilibrium before readings are obtained. Calculation of Thermodynamic Values Construct a plot of E(y axis) versus T(x axis). The instantaneous slope of this plot at any temperature T gives the value of dE/dT at that temperature. Over the temperature range in this experiment, the value of dE/dT is reasonably constant, and the plot of E versus T should give a straight line. Substitution into Equation 4 gives ΔS. The value of E measured at room temperature is used to determine ΔG of the reaction. E° may be determined by plotting E versus T and obtaining the value at 25℃. The literature value is 1.100V for the cell used in this experiment. ΔG and ΔH for the reaction can then be calculated, as can ΔG° and ΔH°. Note: It is important to realize that the voltage measurements at each temperature should not be taken until the system is at thermal equilibrium. Usually, a period of at least 15 minutes is sufficient for these conditions to be met. No reaction occurs until all connections are made. Connect the wires to the meter last. If a negative deflection occurs on the meter, reverse the wires to the meter. Disconnect one wire during the thermal equilibration so that the concentrations don’t change. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn

Pre-laboratory Questions 1.For the half-reaction Sn+2e-Sn2 Caleulate the cell voltage,E.at 25C if the concentration of Snis 0.IM and that of Snis 0.5M,using the Nerst equation E for this cell is+0.15V. 2.Calculate AG(in kJ)for the reaction Cd+Pb*Cd+Pb If the potential,E,ofthe cell was measuredas+9V. 3.How many moles ofelectrons are involved in the electrochemical oxidation of 118.7g of Sn to Sn?How many faradays ofelectricity are involved? 4.What is the function of a salt bridge in a galvanic cell? Section PDF文件使用"pdfFactory Pro”试用版本创建n,fineprint,com,cn
Pre-laboratory Questions: 1. For the half-reaction 4 2 Sn 2e Sn + - + + Calculate the cell voltage, E, at 25℃ if the concentration of Sn4+ is 0.1M and that of Sn4+ is 0.5M, using the Nerst equation. E° for this cell is +0.15V. 2. Calculate ΔG (in kJ) for the reaction 2 2 Cd Pb Cd Pb + + + + If the potential, E, of the cell was measured as +0.29V. 3. How many moles of electrons are involved in the electrochemical oxidation of 118.7g of Sn2+ to Sn4+? How many faradays of electricity are involved? 4. What is the function of a salt bridge in a galvanic cell? Name:_______________________________________________________________________ Data:___________________ Section:______________________________________________ PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Data sheet Experiment Data Temperature(C) Voltage(my) Measurement Measurement 2 Measurement 3 Measurement 4 Measurement 5 Calculations 1.Generate a plot of E vs.T.as discussed in the experiment,and determine the value of dE/dTand B 2.Determine the value of As(using at 25C): 3.Calculate the value of AG(usingat 25C): 4.Calculate the value of△fP(using E°at25c) Name: Data Section: PDF文件使用”pdfFactory Pro”试用版本创建ww,fineprint,com,c边
Data sheet Experiment Data ____________________________________________________________ Temperature (℃) Voltage (mv) ____________________________________________________________ Measurement 1 ______________ _____________ Measurement 2 ______________ _____________ Measurement 3 ______________ _____________ Measurement 4 ______________ _____________ Measurement 5 ______________ _____________ Calculations 1. Generate a plot of E vs. T, as discussed in the experiment, and determine the value of dE/dT and E°. 2. Determine the value of ΔS° (using E° at 25℃): 3. Calculate the value of ΔG° (using E° at 25℃): 4. Calculate the value of ΔH° (using E° at 25℃): Name:_______________________________________________________________________ Data:___________________ Section:______________________________________________ PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Questions 1.The cell used in this experiment is known as a galvanic cell.What is the main feature tha distinguishes this cell from one of the electrolytic type? 2.In a galvanic cell,what happens at the anode?At the cathode?What is the direction of election flow?Explain. 3.Atypical set of data were recorded for a Cu-Pb cell like the cell described in this experiment Temp(C) Voltage (E) 265 0.465 0.454 80.0 0.493 Calculate△S,△Gand△for this system at26.5℃. 4.From the results of this experiment,is the reaction spontaneous?How can you tell?In which direction is it spontaneous?Explain. Name: Data: Section: PDF文件使用"pdfFactory Pro”试用版本创建wnw,fineprint,com,cn
Questions 1. The cell used in this experiment is known as a galvanic cell. What is the main feature that distinguishes this cell from one of the electrolytic type? 2. In a galvanic cell, what happens at the anode? At the cathode? What is the direction of election flow? Explain. 3. A typical set of data were recorded for a Cu-Pb cell like the cell described in this experiment. ________________________________ Temp (℃) Voltage (E) ________________________________ 26.5 0.465 2.5 0.454 80.0 0.493 Calculate ΔS, ΔG and ΔH for this system at 26.5℃. 4. From the results of this experiment, is the reaction spontaneous? How can you tell? In which direction is it spontaneous? Explain. Name:_______________________________________________________________________ Data:___________________ Section:______________________________________________ PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn