
The First Law of Thermodynamics Tutorial Lecture PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint..com,cn
1 The First Law of Thermodynamics Tutorial Lecture PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn f
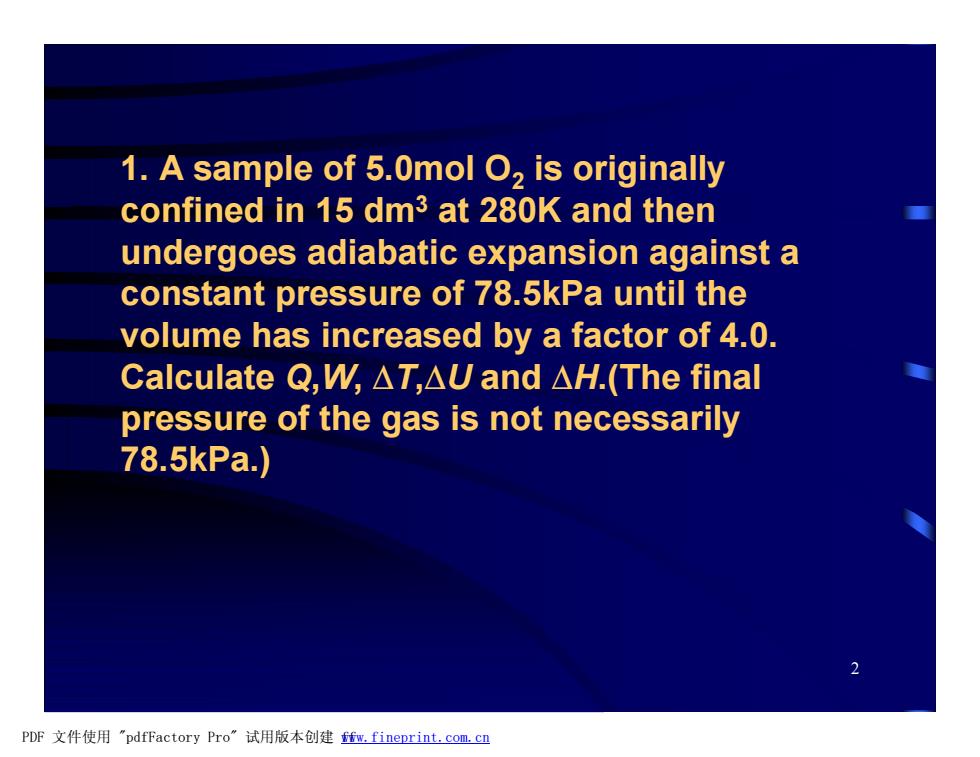
1.A sample of 5.0mol O,is originally confined in 15 dm3 at 280K and then undergoes adiabatic expansion against a constant pressure of 78.5kPa until the volume has increased by a factor of 4.0. Calculate Q,W,△T,△Uand△H.(The final pressure of the gas is not necessarily 78.5kPa) PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
2 1. A sample of 5.0mol O2 is originally confined in 15 dm3 at 280K and then undergoes adiabatic expansion against a constant pressure of 78.5kPa until the volume has increased by a factor of 4.0. Calculate Q,W, DT,DU and DH.(The final pressure of the gas is not necessarily 78.5kPa.) PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

2.A sample consisting of 2.5 mol of perfect gas at 220K and at 200kPa is compressed reversibly and adiabatically until the temperature reaches 255K.Given that its molar constant-volume heat capacity is 27.6JK-1mor1,calculate Q,W,△U,△Hand the final pressure and volume. PDF文件使用"pdfFactory Pro”试用版本创建ffm,fineprint.com,cn
3 2. A sample consisting of 2.5 mol of perfect gas at 220K and at 200kPa is compressed reversibly and adiabatically until the temperature reaches 255K. Given that its molar constant-volume heat capacity is 27.6JK-1mol-1 ,calculate Q,W, DU ,DH and the final pressure and volume. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f
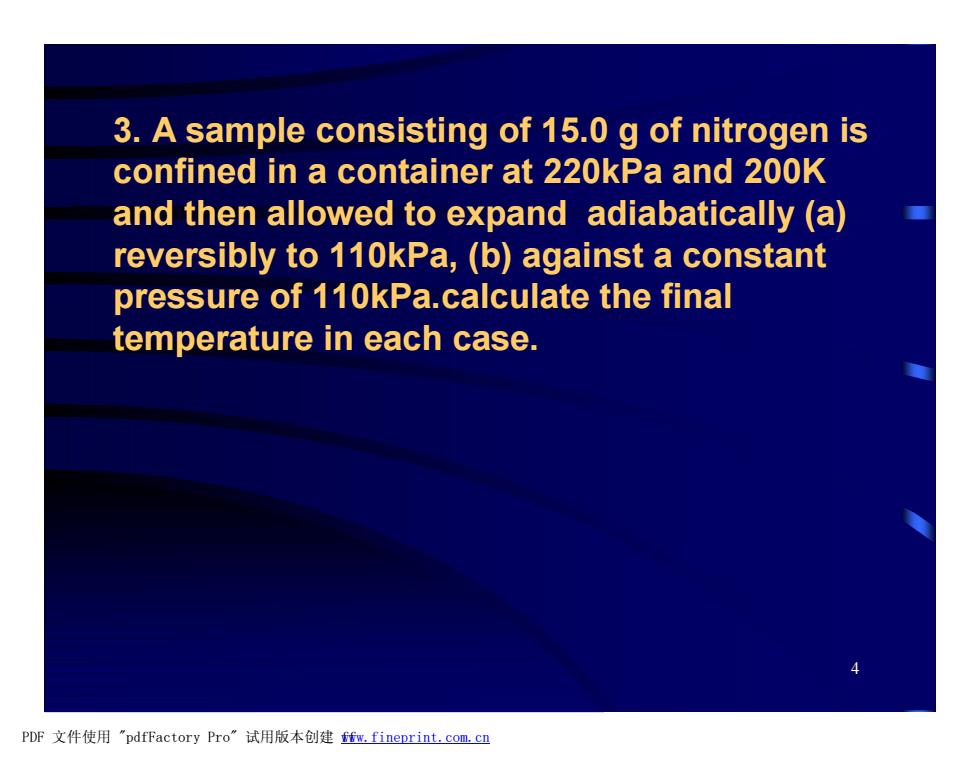
3.A sample consisting of 15.0 g of nitrogen is confined in a container at 220kPa and 200K and then allowed to expand adiabatically (a) reversibly to 110kPa,(b)against a constant pressure of 110kPa.calculate the final temperature in each case. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
4 3. A sample consisting of 15.0 g of nitrogen is confined in a container at 220kPa and 200K and then allowed to expand adiabatically (a) reversibly to 110kPa, (b) against a constant pressure of 110kPa.calculate the final temperature in each case. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

4.When a system is taken from state A to state B along the path ACB in following Fig,80J of heat flows into the system and the system does 30J of work.(a)How much heat flows into the system along path ADB if the work done is10J? (b)When the system is returned from state B to A along the curved path,the work done on the system is 20J.Does the system absorb or liberate heat,and how much?If Up- UA=40J,find the heat absorbed in the processes AD and DB. B D Volume,V 5 PDF文件使用"pdfFactory Pro”试用版本创建ffm,fineprint.com,cn
5 4.When a system is taken from state A to state B along the path ACB in following Fig, 80J of heat flows into the system and the system does 30J of work.(a) How much heat flows into the system along path ADB if the work done is10J? (b)When the system is returned from state B to A along the curved path, the work done on the system is 20J. Does the system absorb or liberate heat, and how much? © If UD - UA=40J, find the heat absorbed in the processes AD and DB. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f