
Test one L填空题 1.在一真空容器中加入NH)2CO(S,将发生下列反应:NH2CO(S)=2NH(g)+C0(g) +H,0(g),则该系统的S=:C=;F=。设各气体均为理想气体,平衡 时总压为p时,其平衡常数K= 2.己知298K,标准压力下萘的燃烧反应为C1oHg(S)+1202(g)→10C02(g)+4H,0(0, 若恒容燃烧热Qv.m=-143.9kJ·mol',则反应的△Hm为_ k·molr;若已知 △HmH0,0=-285.8·mo,△HmC02,g)=-393.5k·molr,则萘的标准摩尔生成 焓为」 3.1mol理想气体在25℃时,恒温可逆地从1dm3膨胀到20dm3,此系统的嫡变△S 。若此气体绝热不可逆从1dm膨胀到20dm3,环境的熵变△S环= 5.2×103 4.若某反应的nK与T的关系式如下:nK= +C,则该反应的 T/K △Hm9=」 A.Cpm=_ 5.理想气体化学反应A(g)+B(g)→C(g,在温度为T、体积为V的密闭容器中达平衡, 若在恒温下向容器中注入n摩尔惰性气体,则该反应的K将 (填变大、变小 或不变),平衡将■ 移动。(填向左、向右或不 6.液相完全互溶的A,B二组分系统,在X=0.6处平衡蒸气压有最高值,则组成为 =0.8的液体在气液平衡时,气相组成y,液相组成X和总组成xM的大小顺序 为 ;将xB=0.8的液体进行精馏,塔底将得到 7.2mol理想气体在298.15K时,压力由200kPa变化到100kPa,则系统的化学势将 。(填:增大、减少或不变)。 8.苯的沸点升高常数K=2.58K·kg·mo厂。若在纯苯液体中加入少量的联苯,溶液的 沸点比纯苯的沸点高了1.15K,则溶液中联苯的量为」 mol.kg。 Ⅱ.选择题:(将答案填在下面的表格中) 题号1234 5678 答案 1.热力学第一定律的数学表达式△U=Q+W只能适用于: A.理想气体B.隔离系统C.封闭系统D.散开系统 2.在一个绝热的刚壁容器中,发生一个化学反应,使系统的温度从T升高到T2,压力 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
-1- Test one I. 填空题 1.在一真空容器中加入(NH4)2CO3(s),将发生下列反应:(NH4)2CO3(s) = 2NH3(g) + CO2(g) + H2O(g),则该系统的 S = ;C = ;F = 。设各气体均为理想气体,平衡 时总压为 p q时,其平衡常数 K q = 。 2.已知 298K,标准压力下萘的燃烧反应为 C10H8 (s) + 12O2 (g) ® 10CO2 (g) + 4H2O (l), 若恒容燃烧热 QV,m = –5143.9 kJ × mol–1,则反应的DrHm q为 kJ × mol–1;若已知 DfHm q (H2O , l) = –285.8 kJ × mol–1,DfHm q (CO2 , g) = –393.5 kJ × mol–1,则萘的标准摩尔生成 焓为 。 3.1mol 理想气体在 25℃时,恒温可逆地从 1dm3 膨胀到 20 dm3,此系统的熵变DS = 。若此气体绝热不可逆从 1dm3膨胀到 20 dm3,环境的熵变DS 环 = 。 4.若某反应的 lnKq与 T 的关系式如下: C T K K + ´ = - / 5.2 10 ln 3 q ,则该反应的 DrHm q = ,DrCp,m = 。 5.理想气体化学反应 A (g) + B (g) ® C (g),在温度为 T、体积为 V 的密闭容器中达平衡, 若在恒温下向容器中注入 n 摩尔惰性气体,则该反应的 K q将 (填变大、变小 或不变),平衡将 移动。(填向左、向右或不) 6.液相完全互溶的 A,B 二组分系统,在 xB = 0.6 处平衡蒸气压有最高值,则组成为 xB = 0.8 的液体在气液平衡时,气相组成 yB,液相组成 xB 和总组成 xM 的大小顺序 为 ;将 xB = 0.8 的液体进行精馏,塔底将得到 。 7.2mol 理想气体在 298.15K 时,压力由 200 kPa 变化到 100 kPa,则系统的化学势将 (填:增大、减少或不变)。 8.苯的沸点升高常数 KB = 2.58K × kg × mol–1。若在纯苯液体中加入少量的联苯,溶液的 沸点比纯苯的沸点高了 1.15K,则溶液中联苯的量为 mol × kg–1。 II. 选择题:(将答案填在下面的表格中) 题号 1 2 3 4 5 6 7 8 答案 1.热力学第一定律的数学表达式 DU = Q + W 只能适用于: A.理想气体 B.隔离系统 C.封闭系统 D.敞开系统 2.在一个绝热的刚壁容器中,发生一个化学反应,使系统的温度从 T1 升高到 T2,压力 PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

从p升高到p2,则: A.Q>0,W>0,AU0,W=0,AU>0 C.Q=0,W>0,AU0,当该反应达平衡时,若要该反应 的平衡向产物方向移动,采取的措施为: A.升高温度和增大压力 B.降低温度和降低压力 C.升高温度和降低压力 D.降低温度和增大压力 4.液态水在373K及101.325kPa下气化成同温同压下的水蒸汽,则该过程: A.△H=0 B.△S=0 C.△G=0 D.△A=0 5.下列各式中那个表示化学势? (aU A. onB)T.pne D.)ryn 6.二组分理想液态混合物总的蒸气压是: A.与溶液的组成无关 B.大于任一纯组分的饱和蒸气压 C.介于两纯组分饱和蒸气压之间D.小于任一纯组分的饱和蒸气压 人在单组分系统的时论中,号出克拉佩龙方程,即号一兴。下面对这公式的描述中, AH 哪点是不予考虑的? A.公式只适用于纯物质的任何两相间的平衡 B.两相中有一相必须是气相 C.公式中△H和△V必须是对应同样量的物质 D.公式表示相变温度随平衡压力的变化情况 8.在723K的抽空容器中将氮和氢以N2:H=1:2的比例通入,使反应N2(g)+3H2(g) 2NH(g)达平衡,则系统的C与F是: A.C=3,F=2 B.C=1,F=1C.C-2,F=21 D.C=2,F=1 III.The normal boiling point of CHsCHa(1)is 111C at p Two mole of CHsCHa(1)at 111C and pis vaporized against Px=0 to its vapour of 111C and p,and then the vapour is heated at constant pressure to the final temperature of2ll℃.Calculate Q,W,△U,△H,△Sand△G of the total process. Given:at normal boiling point,A(CHsCH3)=33.319 kJ.mol For CHCHa(g) PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
-2- 从 p1升高到 p2,则: A.Q > 0,W > 0,DU 0,W = 0,DU > 0 C.Q = 0,W > 0,DU 0,当该反应达平衡时,若要该反应 的平衡向产物方向移动,采取的措施为: A.升高温度和增大压力 B.降低温度和降低压力 C.升高温度和降低压力 D.降低温度和增大压力 4.液态水在 373K 及 101.325kPa 下气化成同温同压下的水蒸汽,则该过程: A.DH = 0 B.DS = 0 C.DG = 0 D.DA = 0 5.下列各式中那个表示化学势? A. T p nc nB U , , ÷ ÷ ø ö ç ç è æ ¶ ¶ B. T V nc nB G , , ÷ ÷ ø ö ç ç è æ ¶ ¶ C. T p nc nB H , , ÷ ÷ ø ö ç ç è æ ¶ ¶ D. T V nc nB A , , ÷ ÷ ø ö ç ç è æ ¶ ¶ 6.二组分理想液态混合物总的蒸气压是: A.与溶液的组成无关 B.大于任一纯组分的饱和蒸气压 C.介于两纯组分饱和蒸气压之间 D.小于任一纯组分的饱和蒸气压 7.在单组分系统的讨论中,导出克拉佩龙方程,即 T V H dT dp D D = 。下面对这公式的描述中, 哪点是不予考虑的? A.公式只适用于纯物质的任何两相间的平衡 B.两相中有一相必须是气相 C.公式中 DH 和 DV 必须是对应同样量的物质 D.公式表示相变温度随平衡压力的变化情况 8.在 723K 的抽空容器中将氮和氢以 N2 : H2 = 1 : 2 的比例通入,使反应 N2 (g) + 3H2 (g) = 2NH3 (g)达平衡,则系统的 C 与 F 是: A.C = 3,F = 2 B.C = 1,F = 1 C.C = 2,F = 21 D.C = 2,F = 1 III. The normal boiling point of C6H5CH3(l) is 111℃ at p q . Two mole of C6H5CH3(l) at 111℃ and p q is vaporized against Pex=0 to its vapour of 111℃ and p q , and then the vapour is heated at constant pressure to the final temperature of 211℃. Calculate Q,W,DU,DH,DS and DG of the total process. Given: at normal boiling point, q DvapHm (C6H5CH3)=33.319 kJ × mol–1 . For C6H5CH3(g) PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

at 111C,=320.77 J.K-.mol and C=140.08 J.K-.mol-.The CH;CH(g)can be regarded as an ideal gas.Compared with V(g).V(1)0. VI.A real gas has the following equation of state:pVm=RT+bp (b is a constant) 1.Prove: (au =0 2.A mole of the real gas is expanded isothermally from Vito V2,Calculate AS 3.Show the relation of and discuss the change of temperature after ap H throttle expansion V.For the reaction C4Hs(g)=CHo(g)+H2(g) the following data at 298.15K are known △H/(kJ.mol) So/(J.molK) C.Hs(g) -0.125 305.3 C,Ho(g) 11006 278.5 H2(g) 0 130.6 (a)What is the△,Hm and△Smof this reaction at298.l5K? (b)What is the A G and K of this reaction at 298.15K? (c)What is the equilibrium constant K if the temperature is 830.15K andH is independent of temperature in the range given? (d)If an inert gas of HO(g)is pumped in the system at the beginning of the reaction,the degree of dissociation of CHs(g)will be increased.Assume the mole ratio of C.H(g): H2O(g)=1:15,calculate the degree of dissociation of CHs(g)at 830.5K and 202.65kPa IV.AB is a binary system of solid-liquid phase.A and Bcan produce a compound C which has incongruent melting and the composition of W%.The table below gives the break and halt temperatures found in the cooling curves of two metals A and B. 3 PDF文件使用"pdfFactory Pro”试用版本创建耀w.fineprint.com.cn
-3- at 111℃, q m S = 320.77 J × K–1 × mol–1 and Cp,m=140.08 J × K–1 × mol–1 . The C6H5CH3(g) can be regarded as an ideal gas. Compared with V(g), V(l)≈0. VI. A real gas has the following equation of state : pVm = RT + bp (b is a constant) 1.Prove: ÷ = 0 ø ö ç è æ ¶ ¶ V T U 2.A mole of the real gas is expanded isothermally from V1 to V2 , Calculate DS. 3.Show the relation of μJ-T = H p T ÷ ÷ ø ö ç ç è æ ¶ ¶ and discuss the change of temperature after throttle expansion. V. For the reaction C4H8(g)= C4H6(g)+H2(g) the following data at 298.15K are known. ΔfH q m/(kJ.mol-1) S q m/(J.mol-1.K-1) C4H8(g) -0.125 305.3 C4H6(g) 110.06 278.5 H2(g) 0 130.6 (a) What is the ΔrH q m and ΔrS q m of this reaction at 298.15K? (b) What is theΔrG q m and Kq of this reaction at 298.15K? (c) What is the equilibrium constant Kq if the temperature is 830.15K andΔrH q m is independent of temperature in the range given? (d) If an inert gas of H2O(g) is pumped in the system at the beginning of the reaction, the degree of dissociation of C4H8(g) will be increased. Assume the mole ratio of C4H8(g) : H2O(g)=1:15, calculate the degree of dissociation of C4H8(g) at 830.5K and 202.65kPa. IV. AB is a binary system of solid-liquid phase. A and B can produce a compound C which has incongruent melting and the composition of WB=70%. The table below gives the break and halt temperatures found in the cooling curves of two metals A and B. PDF 文件使用 "pdfFactory Pro" 试用版本创建 耀www.fineprint.com.cn
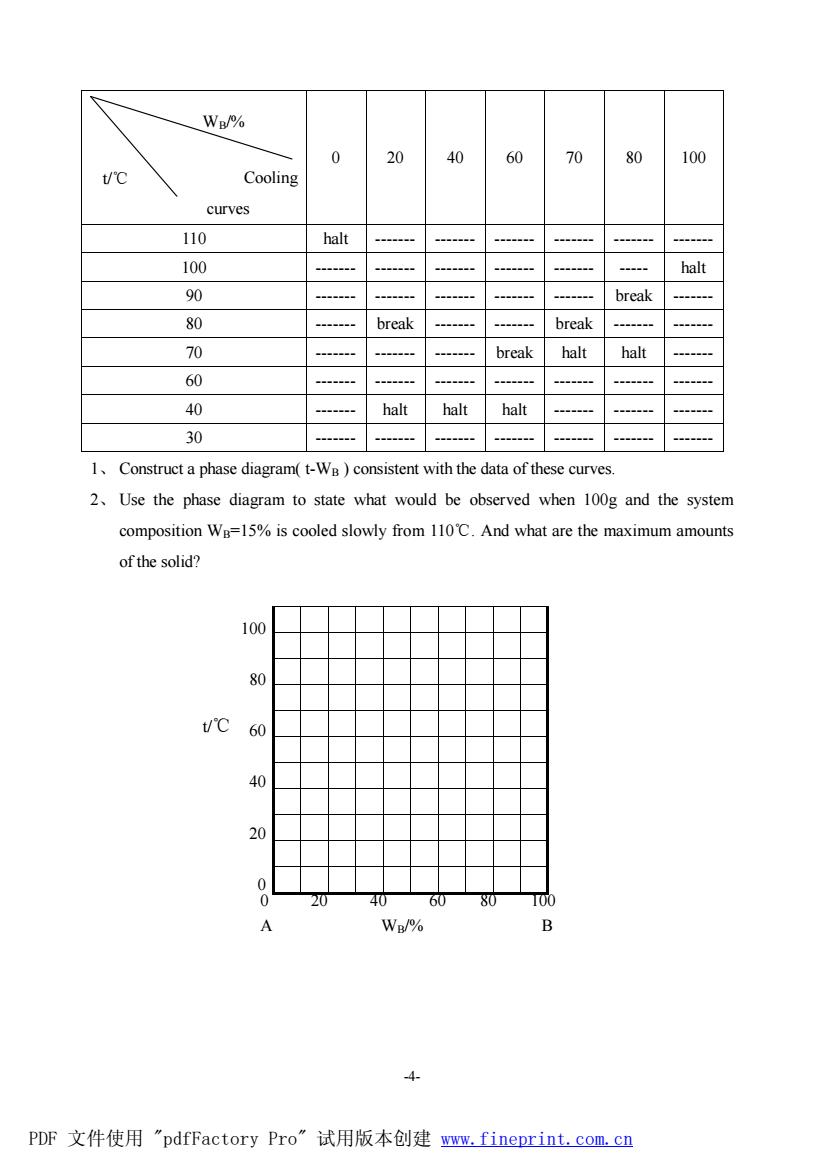
Wp/% 40 80 100 Cooling curves 110 halt 100 90 break 80 break break 70 break halt halt 60 0 halt halt halt 30 1.Construct a phase diagram(t-W)consistent with the data of these curves. 2.Use the phase diagram to state what would be observed when 100g and the system composition We=15%is cooled slowly from 110C.And what are the maximum amounts of the solid? 80 60 24 60 A Wp/% 4 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
-4- Cooling curves 0 20 40 60 70 80 100 110 halt ------- ------- ------- ------- ------- ------- 100 ------- ------- ------- ------- ------- ----- halt 90 ------- ------- ------- ------- ------- break ------- 80 ------- break ------- ------- break ------- ------- 70 ------- ------- ------- break halt halt ------- 60 ------- ------- ------- ------- ------- ------- ------- 40 ------- halt halt halt ------- ------- ------- 30 ------- ------- ------- ------- ------- ------- ------- 1、 Construct a phase diagram( t-WB ) consistent with the data of these curves. 2、 Use the phase diagram to state what would be observed when 100g and the system composition WB=15% is cooled slowly from 110℃. And what are the maximum amounts of the solid? t/℃ 100 80 60 40 20 0 0 20 40 60 80 100 A WB/% B WB/% t/℃ PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn