
A New Type of Lithium Salt Used as Electrolyte Salt of Lithium Ion battery--- Lithium Bis (oxalate)borate PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
A New Type of Lithium Salt Used as Electrolyte Salt of Lithium Ion battery--- Lithium Bis (oxalate) borate PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Presentation on Lithium battery Made by Zhong Qiu,Lan Jinyao, Zhong Xiaotu,Zhong Min. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,c里
Presentation on Lithium battery Presentation on Lithium battery Made by Zhong Qiu, Lan Jinyao, Zhong Xiaotu, Zhong Min. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

1.Comparison of the electrochemical properties LiBOB and LiPFs in electrolytes for LiMn2O4/Li cells 1.1 LiPF6 Spinel lithium manganese oxide (LiMn2Oa)is considered a promising cathode material for lithium secondary batteries,but its poor cyclic ability,especially at high temperatures(above 55 C),remains a problem.One of the factors is related to spinel dissolution-the electrolyte salt LiPF will decompose at elevated temperatures. HF is generated as a product of the decomposing reaction and will dissolve manganese. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
1. Comparison of the electrochemical properties of LiBOB and LiPF6 in electrolytes for LiMn2O4/Li cells 1.1 LiPF6 Spinel lithium manganese oxide (LiMn2O4 ) is considered a promising cathode material for lithium secondary batteries, but its poor cyclic ability, especially at high temperatures (above 55 ℃), remains a problem. One of the factors is related to spinel dissolution—the electrolyte salt LiPF6 will decompose at elevated temperatures. HF is generated as a product of the decomposing reaction and will dissolve manganese. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

1.2 LiBOB As a lithium salt,lithium bis (oxalato)borate (LiBOB)has some unique properties that no other lithium salt possesses.It can effectively stabilize graphite structure in pure propylene carbonate (PC).The lithium-ion cells containing the LiBOB electrolyte exhibits excellent cycling capability even at 70 C.Moreover,the dissolution of Mn in a LiBOB electrolyte is far lower than in a LiPF6 one.Therefore,it appears that LiBOB is a superior candidate for use in lithium cells with LiMn2O4 cathodes than LiPF6. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cm
1.2 LiBOB As a lithium salt, lithium bis (oxalato) borate (LiBOB) has some unique properties that no other lithium salt possesses. It can effectively stabilize graphite structure in pure propylene carbonate (PC) .The lithium-ion cells containing the LiBOB electrolyte exhibits excellent cycling capability even at 70 ℃. Moreover, the dissolution of Mn in a LiBOB electrolyte is far lower than in a LiPF6 one .Therefore, it appears that LiBOB is a superior candidate for use in lithium cells with LiMn2O4 cathodes than LiPF6 . PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
2.thermal behavior of Li bis (oxalato)bte LiBOB According to thermogravimetric experiments,lithium bis (oxalato)borate is fully stable up to approximately 300 C. Differential thermal analysis combined with thermogravimetric analysis (DTA-TGA) using a scanning rate of 10 C.min 1,showed that LiBOB is stable up to 302 C. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
2. thermal behavior of Li bis (oxalato) borate LiBOB According to thermogravimetric experiments, lithium bis (oxalato) borate is fully stable up to approximately 300 ℃. Differential thermal analysis combined with thermogravimetric analysis (DTA–TGA), using a scanning rate of 10 ℃·min−1 , showed that LiBOB is stable up to 302 ℃. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
Beyond this temperature,it decomposes,rather than melts.This salt is chemically stable in organic solutions,but is reported to slowly decompose by hydrolysis to LiBO2 and LiOOCCOOH Amine et al.presented the thermogravimetric curve for LiBOB in the temperature range of 20-600 C.Yu et al.reported on the stability of LiBOB in the temperature range of 240-300 C,and on LiBOB decomposition to Li2CO3,B2O3,CO2,and CO when the temperature is higher than 300 C.They observed a weight loss related to two processes at temperatures in the region of 320 Cand 450C.It was reported that the decomposition of 2 Cproduces B2O3 and CO2. PDF文件使用"pdfFactory Pro”试用版本创建www.fineprint.com.cn
Beyond this temperature, it decomposes, rather than melts .This salt is chemically stable in organic solutions, but is reported to slowly decompose by hydrolysis to LiBO2 and LiOOCCOOH. Amine et al. presented the thermogravimetric curve for LiBOB in the temperature range of 20–600 ℃. Yu et al. reported on the stability of LiBOB in the temperature range of 240–300 ℃, and on LiBOB decomposition to Li2CO3 , B2O3 , CO2 , and CO when the temperature is higher than 300 ℃. They observed a weight loss related to two processes at temperatures in the region of 320 ℃and 450 ℃. It was reported that the decomposition of LiBOB at 302 ℃produces B2O3 and CO2 . PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
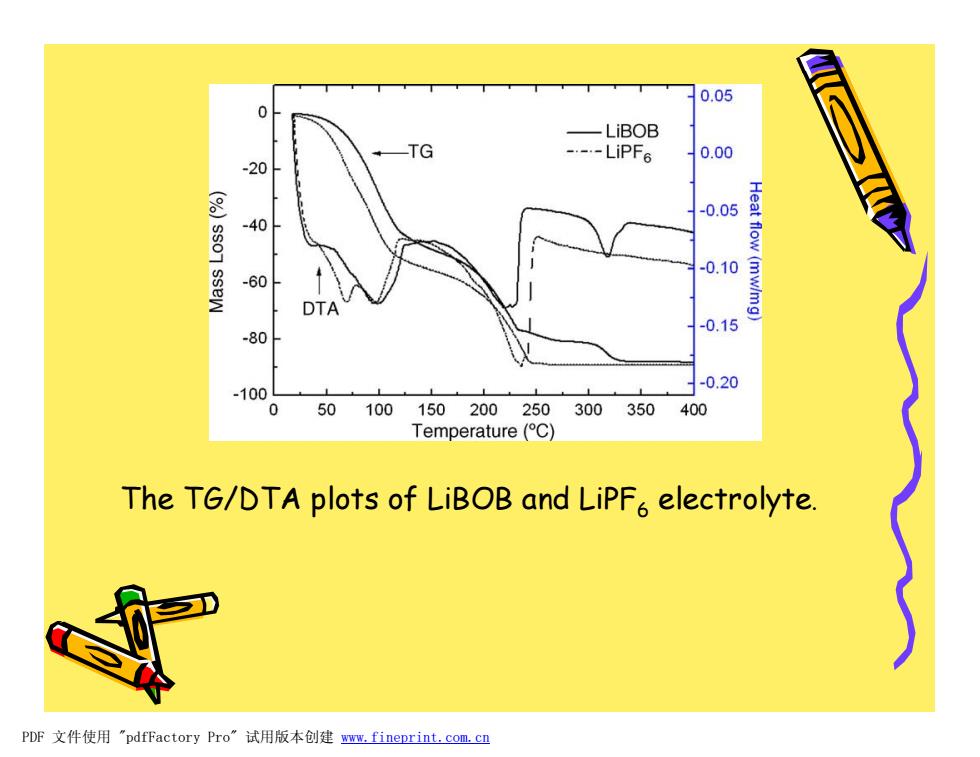
0.05 0 -LiBOB ---LiPF6 0.00 -20 -0.05 -40 0.10 -60 Heat flow(mw/mg) DTA 0.15 -80 -0.20 -100 0 50100150200250300350400 Temperature(C) The TG/DTA plots of LiBOB and LiPFa electrolyte. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
The TG/DTA plots of LiBOB and LiPF6 electrolyte. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

SEM micrographs of:(a)pristine LiBOB samples:(b)Li after heating to 350 C.A scale appears in each microgi PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,c里
SEM micrographs of: (a) pristine LiBOB samples; (b) LiBOB after heating to 350 ℃. A scale appears in each micrograph. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
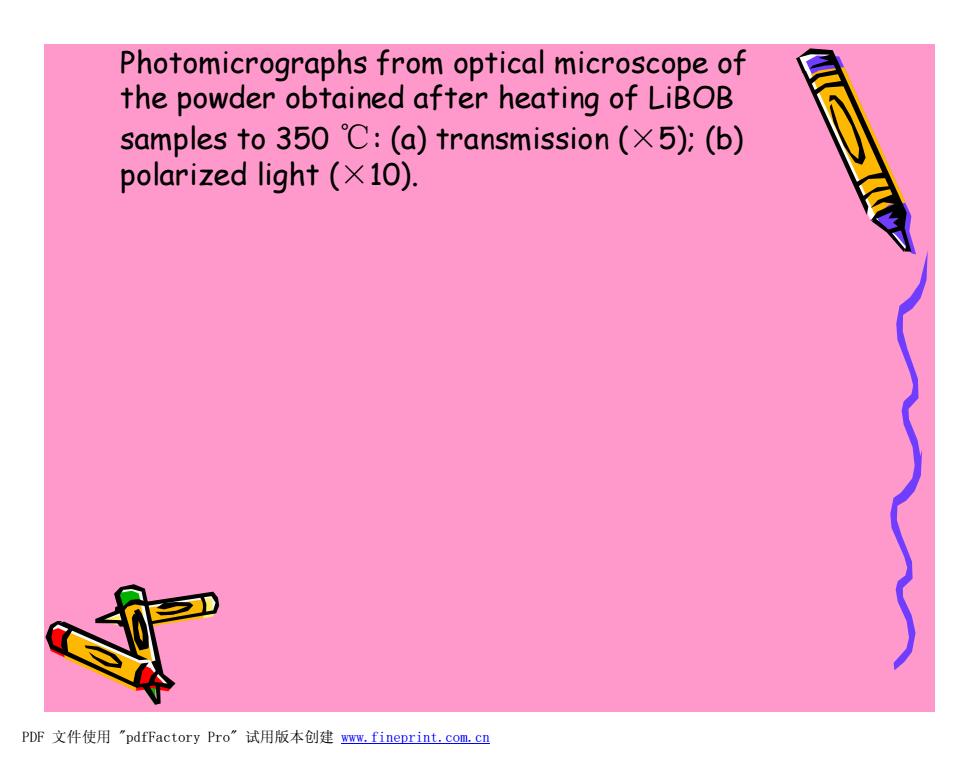
Photomicrographs from optical microscope of the powder obtained after heating of LiBOB samples to350℃:(a)transmission(×5):(b) polarized light (X10). PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
Photomicrographs from optical microscope of the powder obtained after heating of LiBOB samples to 350 ℃: (a) transmission (×5); (b) polarized light (×10). PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

3.The application of LiBOB It is well known that the formation of solid electrolyte interphase (SEI)on the surface of fresh electrodes is an electrochemical process,which is electrochemically initiated by the migration of lithium atoms from the positive electrode to the negative electrode.Therefore,the consumption of lithium due to the formation of SEI can be detected by the change of differential capacity profile (dQ/dV)during formation. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,c四
3.The application of LiBOB It is well known that the formation of solid electrolyte interphase (SEI) on the surface of fresh electrodes is an electrochemical process, which is electrochemically initiated by the migration of lithium atoms from the positive electrode to the negative electrode. Therefore, the consumption of lithium due to the formation of SEI can be detected by the change of differential capacity profile (dQ/dV) during formation. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn