正在加载图片...
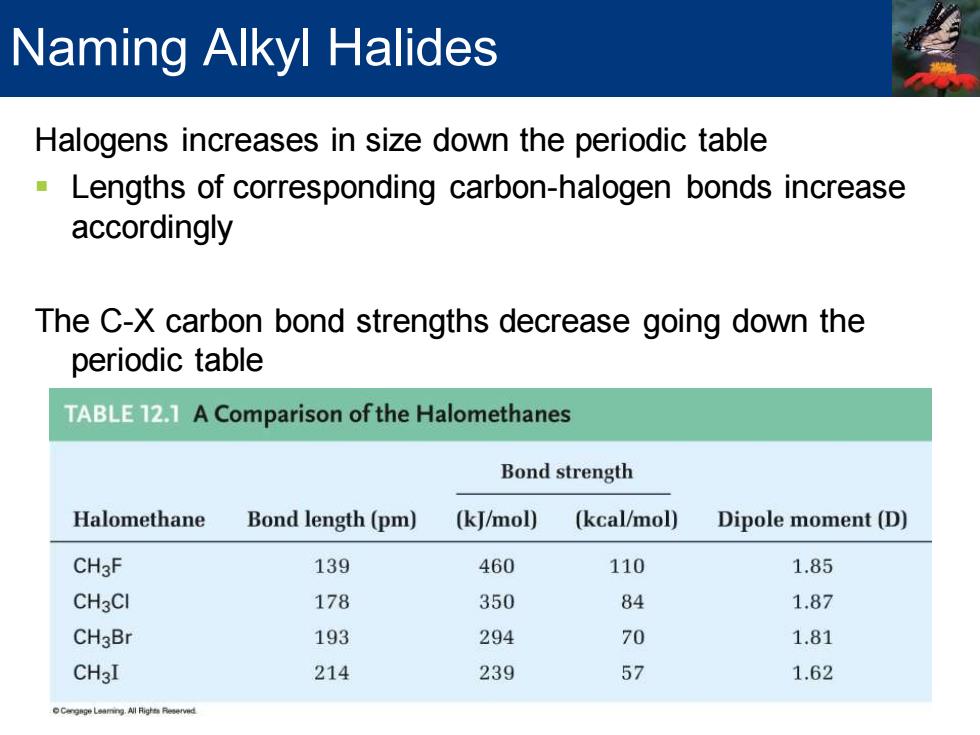
Naming Alkyl Halides Halogens increases in size down the periodic table Lengths of corresponding carbon-halogen bonds increase accordingly The C-X carbon bond strengths decrease going down the periodic table TABLE 12.1 A Comparison of the Halomethanes Bond strength Halomethane Bond length(pm) (kJ/mol) (kcal/mol) Dipole moment(D) CH3F 139 460 110 1.85 CH3CI 178 350 84 1.87 CH3Br 193 294 70 1.81 CH3I 214 239 57 1.62 Halogens increases in size down the periodic table ▪ Lengths of corresponding carbon-halogen bonds increase accordingly The C-X carbon bond strengths decrease going down the periodic table Naming Alkyl Halides