正在加载图片...
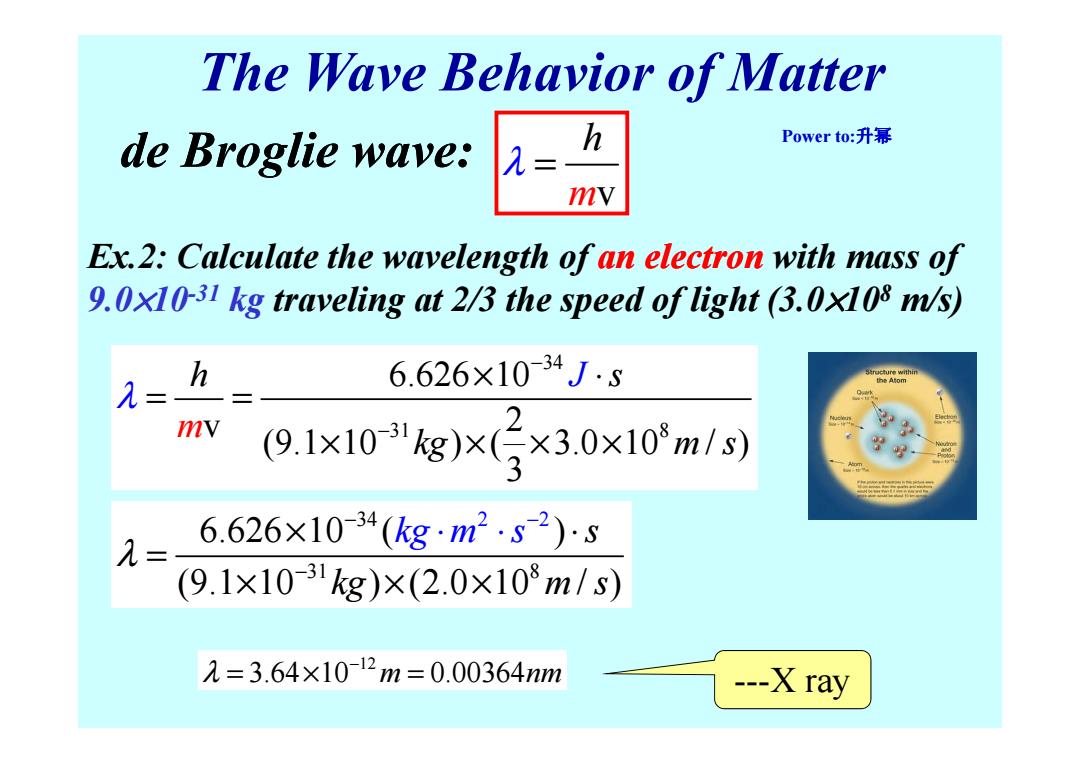
The Wave Behavior of Matter de Broglie wave: h Power to:升幂 = mv Ex.2:Calculate the wavelength of an electron with mass of 9.0x10-31 kg traveling at 2/3 the speed of light (3.0x108 m/s) h 6.626×10-34J5 λ= mv (9.1x10kg)x3×3.0×10m/s) 6.626×10-34(kgm2.82)s (9.1×1031kg)×(2.0×108m/s) 2=3.64×10-12m=0.00364m ---X rayThe Wave Behavior of Matter Ex.2: Calculate the wavelength of an electron with mass of 9.0×10-31 kg traveling at 2/3 the speed of light (3.0×10 8 m/s) m v h de Broglie wave: λ = 34 h s 6.626 10 J λ − × ⋅ = = Power to:升幂 31 8 6.626 10 v 2 (9.1 10 ) ( 3.0 10 / ) 3 h s kg J m m s λ − × ⋅ = = × × × × 34 3 2 2 1 8 6.626 10 ( ) (9.1 10 ) (2.0 10 / ) s kg m m s s kg λ − − − × ⋅ = × × × ⋅ ⋅ 12 λ 3.64 10 0.00364 m nm − = × = ---X ray