
G山东理子大军 Analytical Chemistry Chapter 2 Errors and data treatment in quantitative analysis
Analytical Chemistry Chapter 2 Errors and data treatment in quantitative analysis 2023/7/28 1

G 归东理工大军 Analytical Chemistry Learning Objective u To master the types,characteristics and expression methods of errors; u To master the definitions and applications of the precision and the accuracy,and also to understand the relationship between the precision and the accuracy;
Analytical Chemistry Learning Objective u To master the types, characteristics and expression methods of errors; u To master the definitions and applications of the precision and the accuracy, and also to understand the relationship between the precision and the accuracy; 2023/7/28 2
归东理2大军 Analytical Chemistry u To know about the distribution rules of random error,and master the definitions, functions and calculating methods of confidence levels and confidence intervals; u To know about the evaluation methods of the quantitative data,realize the importance of improving the accuracy of analytical results;
Analytical Chemistry u To know about the distribution rules of random error, and master the definitions, functions and calculating methods of confidence levels and confidence intervals; u To know about the evaluation methods of the quantitative data, realize the importance of improving the accuracy of analytical results; 2023/7/28 3
G 归东龙子大写 Analytical Chemistry u To master the ways and methods for improving the accuracy of analytical results; u To know about the concept of significant figures, and master the rules of the computing and arithmetic rounding off
Analytical Chemistry u To master the ways and methods for improving the accuracy of analytical results; u To know about the concept of significant figures, and master the rules of the computing and arithmetic rounding off. 2023/7/28 4
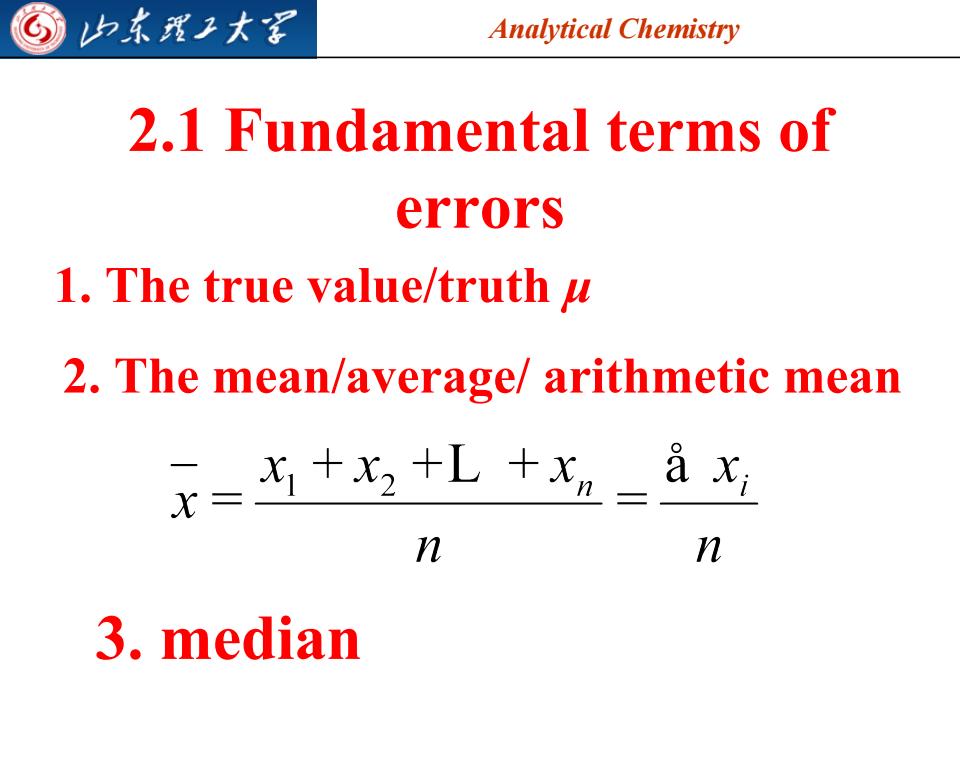
归东理工大军 Analytical Chemistry 2.1 Fundamental terms of errors 1.The true value/truth 2.The mean/average/arithmetic mean x=++L+2=且龙 n n 3,median
Analytical Chemistry 2.1 Fundamental terms of errors 1. The true value/truth μ 2. The mean/average/ arithmetic mean 3. median 2023/7/28 5

G 归东龙子大写 Analytical Chemistry The middle numerical value in a set of values. 4.Accuracy Accuracy describes the nearness of an experimental value to the true value. Accuracy is expressed as error E=x-m relative error (RE)is calculated by the following formula:
Analytical Chemistry 4. Accuracy Accuracy is expressed as error relative error (RE) is calculated by the following formula: The middle numerical value in a set of values. Accuracy describes the nearness of an experimental value to the true value. 2023/7/28 6

归东理工大军 Analytical Chemistry E RE= '100% m 5.Precision precision refers to the agreement between values in a set of date,it describes the reproducibility of measurements. (1)Deviation d;=xi-x
Analytical Chemistry 5. Precision precision refers to the agreement between values in a set of date, it describes the reproducibility of measurements. (1) Deviation 2023/7/28 7
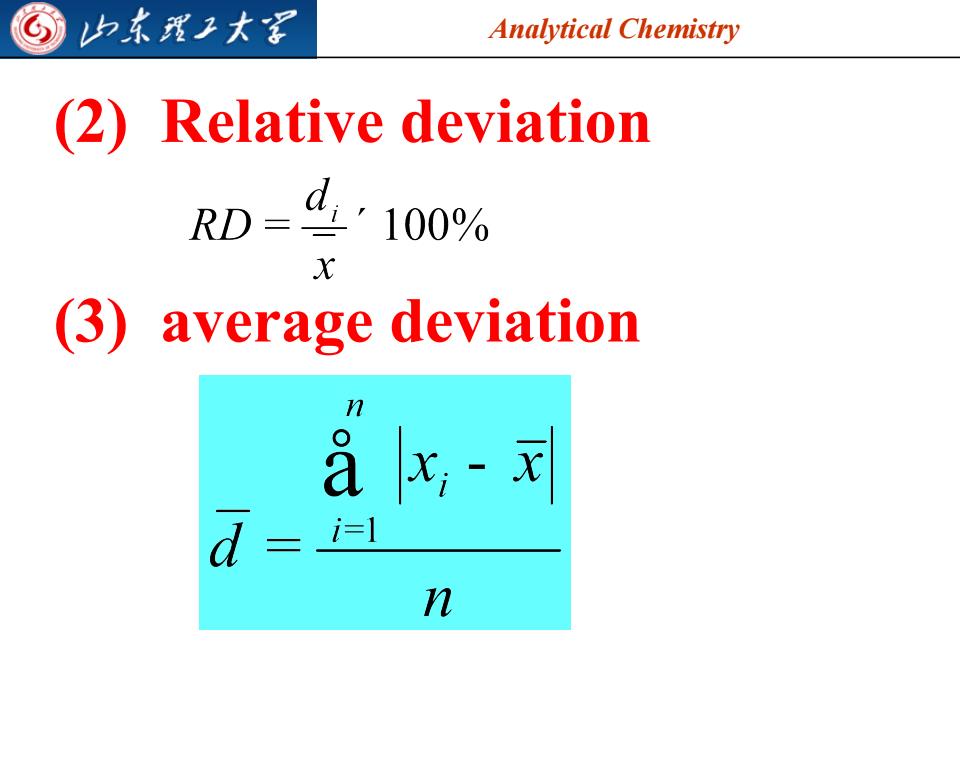
G 归东我王大 Analytical Chemistry (2) Relative deviation RD=4100% X (3)average deviation ax;-x d i=l n
Analytical Chemistry (2) Relative deviation (3) average deviation 2023/7/28 8
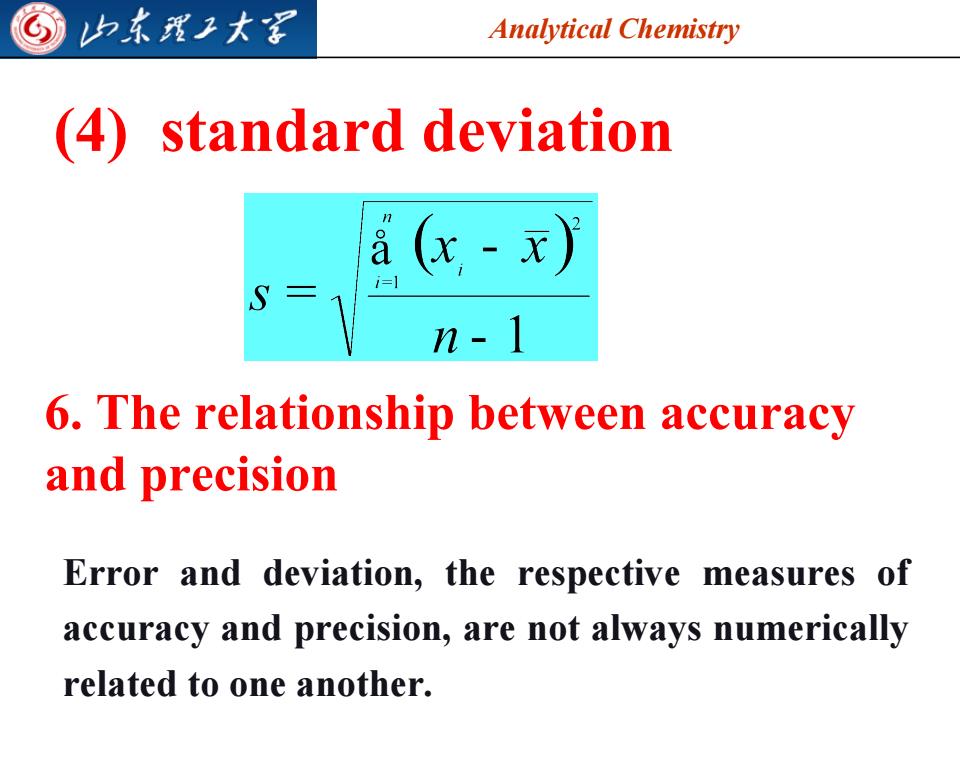
归东我工大军 Analytical Chemistry (4) standard deviation a (x-x)》 i=1 n-1 6.The relationship between accuracy and precision Error and deviation,the respective measures of accuracy and precision,are not always numerically related to one another
Analytical Chemistry (4) standard deviation 6. The relationship between accuracy and precision Error and deviation, the respective measures of accuracy and precision, are not always numerically related to one another. 2023/7/28 9

归东理工大图 Analytical Chemistry That is,the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy
Analytical Chemistry That is, the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy. 2023/7/28 10