正在加载图片...
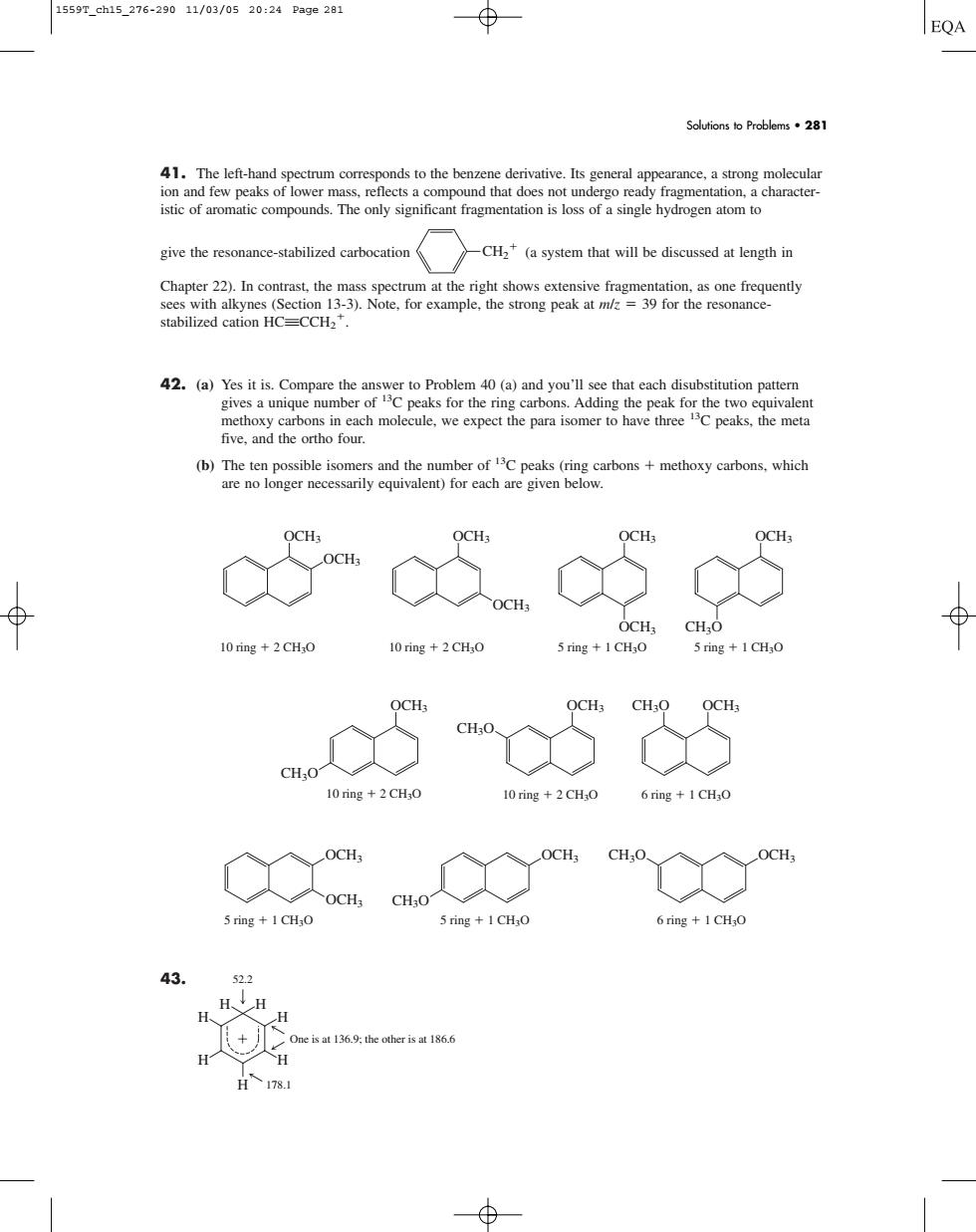
1559r.ch15276-29011/02/0520:24Page281 EQA Solutions to Problems281 41.The left-h characte give the resonance-stabilized carbocation CH(a system that will be discussed at length in stabilized cation HC=CCH2". five.and the ortho four. b c58 CH,CHO 10 ring+2CHO 10ig+2CH,0 5 ring +1CHao 5ring+1CH.O ad "as to CH:O 10 ring +2 CHO 10ring+2CH:o 6ring +1CHO a 5 ring 1 CH,O 5 ring +I CHO 6ring+1CHO +心41. The left-hand spectrum corresponds to the benzene derivative. Its general appearance, a strong molecular ion and few peaks of lower mass, reflects a compound that does not undergo ready fragmentation, a characteristic of aromatic compounds. The only significant fragmentation is loss of a single hydrogen atom to give the resonance-stabilized carbocation (a system that will be discussed at length in Chapter 22). In contrast, the mass spectrum at the right shows extensive fragmentation, as one frequently sees with alkynes (Section 13-3). Note, for example, the strong peak at m/z 39 for the resonancestabilized cation HCqCCH2 . 42. (a) Yes it is. Compare the answer to Problem 40 (a) and you’ll see that each disubstitution pattern gives a unique number of 13C peaks for the ring carbons. Adding the peak for the two equivalent methoxy carbons in each molecule, we expect the para isomer to have three 13C peaks, the meta five, and the ortho four. (b) The ten possible isomers and the number of 13C peaks (ring carbons methoxy carbons, which are no longer necessarily equivalent) for each are given below. 43. H H H H H One is at 136.9; the other is at 186.6 178.1 52.2 H H 5 ring 1 CH3O 5 ring 1 CH3O 6 ring 1 CH3O CH3O CH3O OCH3 OCH3 OCH3 OCH3 10 ring 2 CH3O OCH3 CH3O OCH3 CH3O OCH3 CH3O 10 ring 2 CH3O 6 ring 1 CH3O OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 10 ring 2 CH3O 10 ring 2 CH3O 5 ring 1 CH3O 5 ring 1 CH3O OCH3 CH3O CH2 Solutions to Problems • 281 1559T_ch15_276-290 11/03/05 20:24 Page 281��������������