正在加载图片...
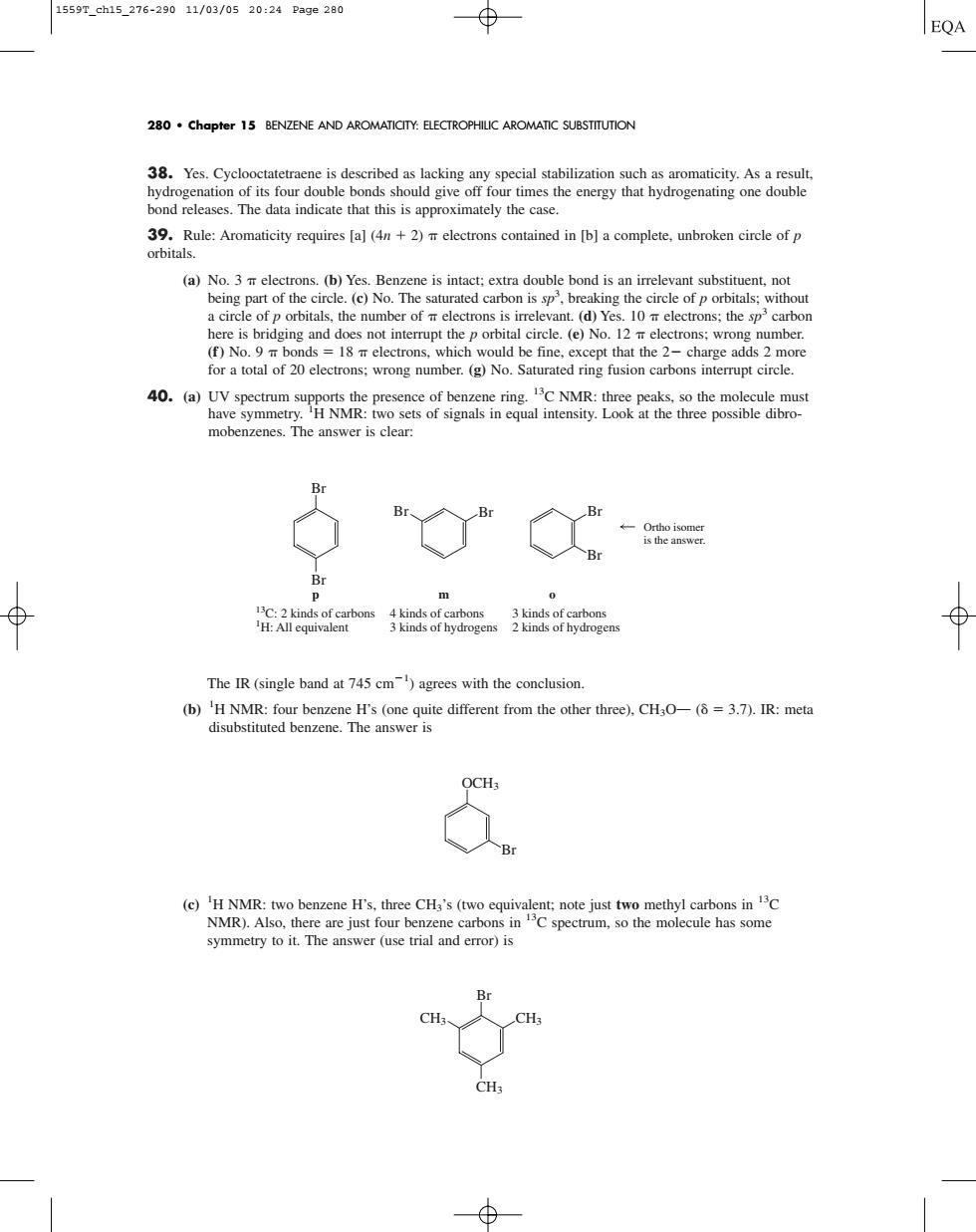
1559T_ch15_276-29011/03/0520:24Pa9e280 EQA 280.Chapter 15 BENZENE AND AROMATICITY:ELECTROPHILIC AROMATIC SUBSTITUTION bond releases.The data indicate that this is approximately the case. 39.Rule:Aromaticity requires [a](4n+)electrons contained in [b]a complete.unbroken circle of p orbitals ene is inta e evant sub 40.) mobenzenes.The answer is clear: The IR(single band at 745 cm)agrees with the conclusion. (h e der re)C OCHs symmetry to it.The answer(use trial and error)is 38. Yes. Cyclooctatetraene is described as lacking any special stabilization such as aromaticity. As a result, hydrogenation of its four double bonds should give off four times the energy that hydrogenating one double bond releases. The data indicate that this is approximately the case. 39. Rule: Aromaticity requires [a] (4n 2) electrons contained in [b] a complete, unbroken circle of p orbitals. (a) No. 3 electrons. (b) Yes. Benzene is intact; extra double bond is an irrelevant substituent, not being part of the circle. (c) No. The saturated carbon is sp3 , breaking the circle of p orbitals; without a circle of p orbitals, the number of electrons is irrelevant. (d) Yes. 10 electrons; the sp3 carbon here is bridging and does not interrupt the p orbital circle. (e) No. 12 electrons; wrong number. (f) No. 9 bonds 18 electrons, which would be fine, except that the 2 charge adds 2 more for a total of 20 electrons; wrong number. (g) No. Saturated ring fusion carbons interrupt circle. 40. (a) UV spectrum supports the presence of benzene ring. 13C NMR: three peaks, so the molecule must have symmetry. 1 H NMR: two sets of signals in equal intensity. Look at the three possible dibromobenzenes. The answer is clear: The IR (single band at 745 cm1 ) agrees with the conclusion. (b) 1 H NMR: four benzene H’s (one quite different from the other three), CH3OO ( 3.7). IR: meta disubstituted benzene. The answer is (c) 1 H NMR: two benzene H’s, three CH3’s (two equivalent; note just two methyl carbons in 13C NMR). Also, there are just four benzene carbons in 13C spectrum, so the molecule has some symmetry to it. The answer (use trial and error) is Br CH3 CH3 CH3 Br OCH3 Br Br 13C: 2 kinds of carbons 1 H: All equivalent 4 kinds of carbons 3 kinds of hydrogens p Br Br m Br o Br Ortho isomer is the answer. 3 kinds of carbons 2 kinds of hydrogens 280 • Chapter 15 BENZENE AND AROMATICITY: ELECTROPHILIC AROMATIC SUBSTITUTION 1559T_ch15_276-290 11/03/05 20:24 Page 280���