正在加载图片...
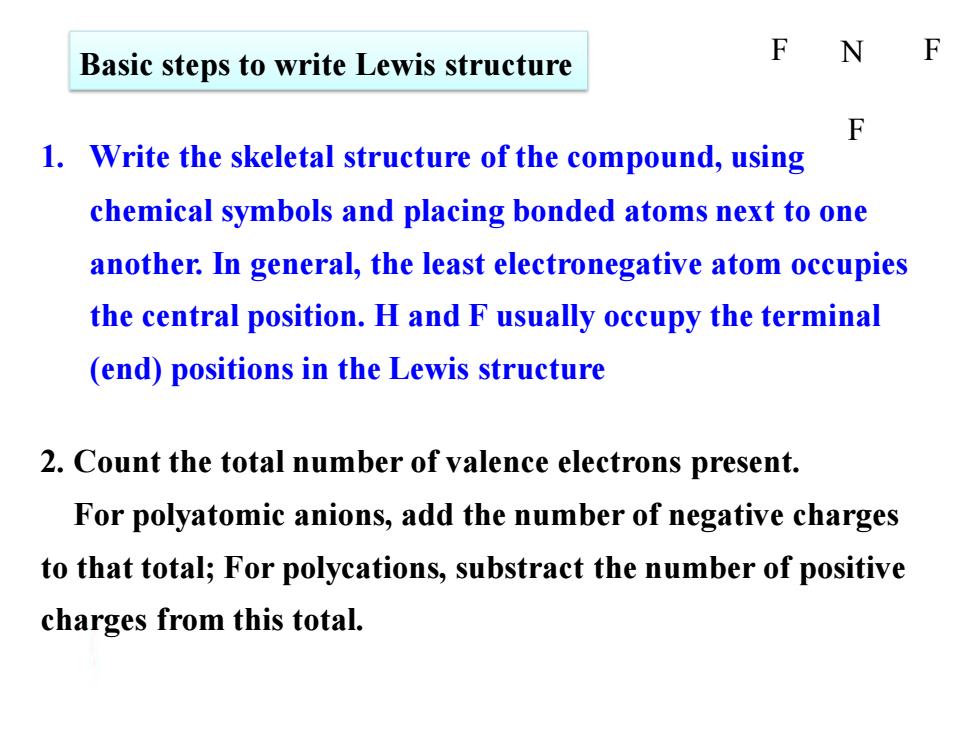
F Basic steps to write Lewis structure N F 1.Write the skeletal structure of the compound,using chemical symbols and placing bonded atoms next to one another.In general,the least electronegative atom occupies the central position.H and F usually occupy the terminal (end)positions in the Lewis structure 2.Count the total number of valence electrons present. For polyatomic anions,add the number of negative charges to that total;For polycations,substract the number of positive charges from this total. Basic steps to write Lewis structure 1. Write the skeletal structure of the compound, using chemical symbols and placing bonded atoms next to one another. In general, the least electronegative atom occupies the central position. H and F usually occupy the terminal (end) positions in the Lewis structure 2. Count the total number of valence electrons present. For polyatomic anions, add the number of negative charges to that total; For polycations, substract the number of positive charges from this total. F N F F